Use the standard reduction potentials (Appendix M) for the half-reactions [AuCl 4 ] (aq) + 3
Question:
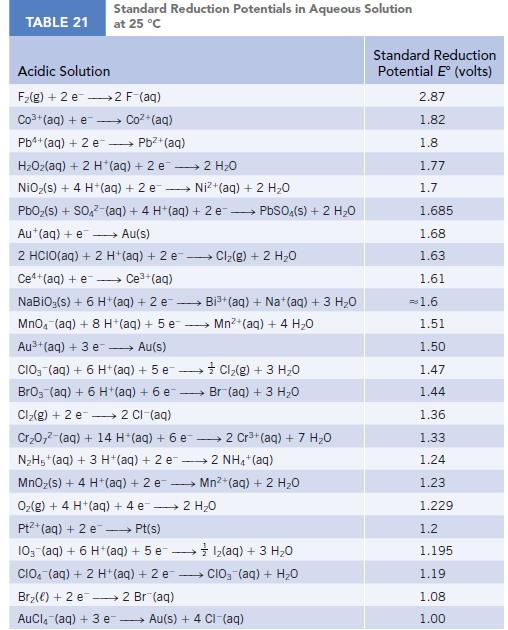
Use the standard reduction potentials (Appendix M) for the half-reactions [AuCl4]−(aq) + 3 e− → Au(s) + 4 Cl−(aq) and Au3+(aq) + 3 e− → Au(s) to calculate the value of Kformation for the complex ion [AuCl4]−(aq).
Data given in Appendix M
Transcribed Image Text:
TABLE 21 Standard Reduction Potentials in Aqueous Solution at 25 °C Acidic Solution F₂(g) + 2 e 2 F-(aq) Co3+ (aq) + e Coz+(aq) Pb4+ (aq) + 2 e - Pb²+(aq) HzOz(aq) + 2 H*(aq) +2e → 2H2O NiO₂(s) + 4 H+ (aq) + 2 e→→→→→→ Ni+(aq) + 2 HO PbO₂ (s) + SO4² (aq) + 4 H+ (aq) + 2e → PbSO4(s) + 2 H₂O Au+ (aq) + e→→→→→ Au(s) 2 HCIO(aq) + 2 H+ (aq) + 2 e-- - Ce+(aq) + e→→→→ Ce³+ (aq) NaBiO;(s) + 6 H+ (aq) + 2 e- → MnO4 (aq) + 8 H+ (aq) + 5 e Au³+ (aq) + 3 e→→→→→ Au(s) CIO (aq) + 6 H+ (aq) +5 e→→→→→→ BrO3 (aq) + 6 H+ (aq) + 6 e- Cl₂(g) + 2 e 2 Cl-(aq) Cr₂0,² (aq) + 14 H*(aq) + 6 e 2 Cr³+ (aq) + 7 H₂O N₂H5+ (aq) + 3 H+ (aq) + 2 e2 NH4+ (aq) MnO₂ (s) + 4 H+ (aq) + 2 e O₂(g) + 4 H+(aq) + 4 e 2 H₂O Pt+ (aq) + 2 e →→→→→ Pt(s) 10- (aq) + 6 H+ (aq) + 5 e1₂(aq) + 3 H₂O CIO (aq) + 2 H+ (aq) + 2 e CIO₂ (aq) + H₂O Br₂() +2 e 2 Br(aq) AuCl(aq) + 3 e → Cl₂(g) + 2 H₂O → Bi³+ (aq) + Na+ (aq) + 3 H₂O Mn²+ (aq) + 4H₂O Cl₂(g) + 3 H₂O Br (aq) + 3 H₂O Mn²+ (aq) + 2 H₂O Au(s) + 4 CI-(aq) Standard Reduction Potential E (volts) 2.87 1.82 1.8 1.77 1.7 1.685 1.68 1.63 1.61 = 1.6 1.51 1.50 1.47 1.44 1.36 1.33 1.24 1.23 1.229 1.2 1.195 1.19 1.08 1.00
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate the formation constant Kf for the complex ion AuCl4aq we need to use the following equa...View the full answer

Answered By

Vineet Kumar Yadav
I am a biotech engineer and cleared jee exam 2 times and also i am a math tutor. topper comunity , chegg India, vedantu doubt expert( solving doubt for iit jee student on the online doubt solving app in live chat with student)
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Use the standard reduction potentials (Appendix M) for the half-reactions [Zn(OH) 4 ] 2 (aq) + 2 e Zn (s) + 4 OH (aq) and Zn 2+ (aq) + 2e Zn(s) to calculate the value of K formation for the...
-
Use the standard reduction potentials to find the equilibrium constant for each of the following reactions at 25°C: (a) (b) (c) Br2(1) + 21-(aq )- 2Br_(aq) + 12(s) 5Fe2 + (aq) +MnO4 (aq ) + 8H +...
-
Use the standard reduction potentials (Appendix M) for the half-reactions Hg 2 Cl 2 (s) + 2e 2 Hg() + 2 Cl (aq) and Hg 2 2+ (aq) + 2 e 2 Hg() to calculate the value of K sp for Hg 2 Cl 2 . Data...
-
Raheem & Co. purchased a fixed asset on 1.4.2018 for Rs.2,50,000. Depreciation is to be provided @10% annually according to the Straight-line method. The books are closed on 31st March every year....
-
A project has estimated annual net cash flows of $150,000. It is estimated to cost $885,000. Determine the cash payback period.
-
You are evaluating two different cookie-baking ovens. The Pillsbury 707 costs $57,000, has a five-year life, and has an annual OCF (after tax) of -$10,000 per year. The Keebler CookieMunster costs...
-
Explain the concept of fiduciary responsibility and ethics as they relate to the general manager of a hospitality operation.
-
Southern Copper Corporation (PCU) acquired mining equipment for $100,000 cash on January 1, 2009. The equipment had an expected useful life of four years and zero salvage value . PCU calculates...
-
1 Delloel Ltd. on the note's maturity date. X EXERCISE 12-3 Baldwin Inc. operates in a jurisdiction that levies two types of sales taxes: a federal, 6%, refundable goods and services tax and a...
-
In 1937, R. Schwartz and M. Schmiesser prepared a yellow-orange bromine oxide (BrO 2 ) by treating Br 2 with ozone in a fluorocarbon solvent. Many years later, J. Pascal found that, on heating, this...
-
The reaction occurring in the cell in which Al 2 O 3 and aluminum salts are electrolyzed is Al 3+ (aq) +3 e Al(s). If the electrolysis cell operates at 5.0 V and 1.0 10 5 A, what mass of aluminum...
-
Are these bonds issued at a discount or a premium? Explain your answer. lo4
-
Find anti derivative of cos x,sin x,sec r,sec r tan r,csc xcotx,csc r
-
Find derivative of arcsin x
-
1. Examine the impact of feedwater heater pressure on cycle efficiency and determine the intermediate pressure for optimal performance for one heater.
-
3. Environmental impact of adding feedwater heaters
-
1. Discuss the importance of genetic diversity in fish populations. 2. a) Habitat restoration and connectivity in population conservation is faced with many challenges. Discuss (5 marks) b)a) ...
-
Consider the coordinate vectors (a) Find w if S is the basis in Exercise 2(a). (b) Find B if S is the basis in Exercise 4. 614
-
On April 29, 2015, Auk Corporation acquires 100% of the outstanding stock of Amazon Corporation (E & P of $750,000) for $1.2 million. Amazon has assets with a fair market value of $1.4 million (basis...
-
This problem concerns the design of a divider for unsigned binary numbers that will divide a 16-bit dividend by an 8-bit divisor to give an 8-bit quotient. Assume that the start signal (ST = 1) is 1...
-
Design a 4 Ã 4 keypad scanner for the following keypad layout. (a) Assuming only one key can be pressed at a time, find the equations for a number decoder given R 3-0 and C 3- 0 , whose output...
-
Four pushbuttons (B 0 , B 1 , B 2 , and B 3 ) are used as inputs to a logic circuit. Whenever a button is pushed, it is debounced, after which the circuit loads the button number in binary into a...
-
ASSIGNMENT QUESTION PURPOSE The purpose of this assignment is to develop learners' ability to discuss the tax incentives given under the short-Term Economic Recovery Plan (PENJANA). REQUIREMENT...
-
Sako Company's Audio Division produces a speaker that is used by manufacturers of various audio products. Sales and cost data on the speaker follow: Selling price per unit on the intermediate market...
-
If a city acquires a new office building under a capital lease agreement, 1. The cost of the building should be recorded in the ___________ 2. The "cost" of the building is computed as ______________...

Study smarter with the SolutionInn App


