Use the standard reduction potentials (Appendix M) for the half-reactions Hg 2 Cl 2 (s) + 2e
Question:
Use the standard reduction potentials (Appendix M) for the half-reactions Hg2Cl2(s) + 2e− → 2 Hg(ℓ) + 2 Cl−(aq) and Hg22+(aq) + 2 e− → 2 Hg(ℓ) to calculate the value of Ksp for Hg2Cl2.
Data given in Appendix M
Transcribed Image Text:
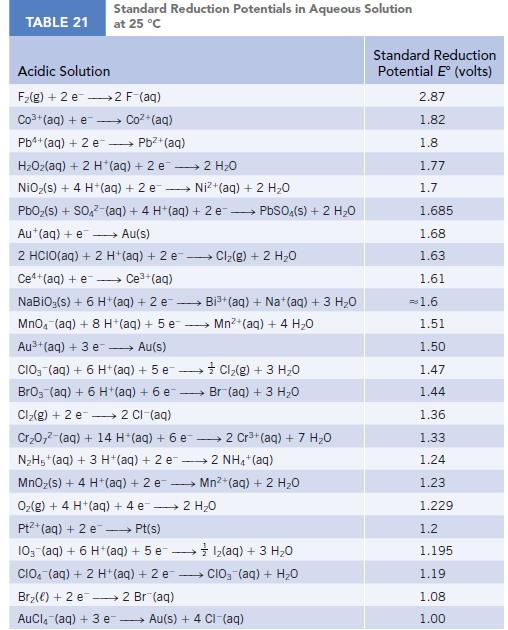
TABLE 21 Standard Reduction Potentials in Aqueous Solution at 25 °C Acidic Solution F₂(g) + 2 e 2 F-(aq) Co3+(aq) + e Coz+(aq) Pb4+ (aq) + 2 e - Pb²+ (aq) HzOz(aq) + 2 H*(aq) +2e → 2H2O NiO₂(s) + 4 H+ (aq) + 2 e→→→→→→ Ni+(aq) + 2 HO PbO₂ (s) + SO4² (aq) + 4 H+ (aq) + 2e → PbSO4(s) + 2 H₂O Au+ (aq) + e→→→→→ Au(s) 2 HCIO(aq) + 2 H+ (aq) + 2 e-- - Ce+(aq) + e→→→→ Ce³+ (aq) NaBiO;(s) + 6 H+ (aq) + 2 e- → MnO4 (aq) + 8 H+(aq) + 5 e Au³+ (aq) + 3 e→→→→→ Au(s) CIO3(aq) + 6 H+ (aq) + 5 e→→→→→→ BrO3 (aq) + 6 H+ (aq) + 6 e- Cl₂(g) + 2 e 2 Cl-(aq) Cr₂0,² (aq) + 14 H*(aq) + 6 e 2 Cr³+ (aq) + 7 H₂O N₂H5+ (aq) + 3 H+ (aq) + 2 e2 NH4+ (aq) MnO₂(s) + 4 H + (aq) + 2 e O₂(g) + 4 H+ (aq) + 4 e 2 H₂O Pt+ (aq) + 2 e →→→→→ Pt(s) 10- (aq) + 6 H+ (aq) + 5 e1₂(aq) + 3 H₂O CIO (aq) + 2 H+ (aq) + 2 e CIO₂ (aq) + H₂O Br₂() +2 e 2 Br(aq) AuCl(aq) + 3 e → Cl₂(g) + 2 H₂O → Bi³+ (aq) + Na+ (aq) + 3 H₂O Mn²+ (aq) + 4 H₂0 Cl₂(g) + 3 H₂O Br (aq) + 3 H₂O Mn²+ (aq) + 2 H₂O Au(s) + 4 CI-(aq) Standard Reduction Potential E (volts) 2.87 1.82 1.8 1.77 1.7 1.685 1.68 1.63 1.61 = 1.6 1.51 1.50 1.47 1.44 1.36 1.33 1.24 1.23 1.229 1.2 1.195 1.19 1.08 1.00
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
Hg2Cl2 2e 2 Hg 0 2 Claq Hg2aq 2e 2 Hg0 E 027 v E0789 v Ecell E oxidation ...View the full answer

Answered By

Sidharth Jain
My name is Sidharth. I completed engineering from National Institute of Technology Durgapur which is one of the top college in India. I am currently working as an Maths Faculty in one of the biggest IITJEE institute in India. Due to my passion in teaching and Maths, I came to this field. I've been teaching for almost 3 years.
Apart from it I also worked as an Expert Answerer on Chegg.com. I have many clients from USA to whom I teach online and help them in their assignments. I worked on many online classes on mymathlab and webassign. I guarantee for grade 'A'.
4.90+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Determine the reactions at the beam supports for the given loading. 50 lb/in 400 lb 12 in 20 in 6 in
-
Use the standard reduction potentials (Appendix M) for the half-reactions [AuCl 4 ] (aq) + 3 e Au(s) + 4 Cl (aq) and Au 3+ (aq) + 3 e Au(s) to calculate the value of K formation for the complex...
-
Use the standard reduction potentials (Appendix M) for the half-reactions [Zn(OH) 4 ] 2 (aq) + 2 e Zn (s) + 4 OH (aq) and Zn 2+ (aq) + 2e Zn(s) to calculate the value of K formation for the...
-
Thor Bhd. (Thor) is a listed company in Malaysia, specializes in selling batteries. At 31 December 2021, Thor holds four distinct types of batteries in its warehouse. The accountant of Thor provided...
-
Determine the average rate of return for a project that is estimated to yield total income of $72,000 over three years, has a cost of $125,000, and has a $25,000 residual value.
-
HotFoot Shoes would like to maintain their cash account at a minimum level of $25,000, but expect the standard deviation in net daily cash flows to be $2,000, the effective annual rate on marketable...
-
Compose a letter to a potential lender addressing only the issue of why the organizational and business structures you have selected for your new business group makes it advantageous for the lender...
-
The records of Grade A Steak Company list the following selected accounts for the quarter ended April 30, 2012: Requirements 1. Prepare a multi-step income statement. 2. M. Davidson, manager of the...
-
In accrual-basis accounting, several transactions must be recorded to reflect all expenses and revenue for the period; which accounts are required to be adjusted or reconciled for the period? Discuss...
-
In 1937, R. Schwartz and M. Schmiesser prepared a yellow-orange bromine oxide (BrO 2 ) by treating Br 2 with ozone in a fluorocarbon solvent. Many years later, J. Pascal found that, on heating, this...
-
The reaction occurring in the cell in which Al 2 O 3 and aluminum salts are electrolyzed is Al 3+ (aq) +3 e Al(s). If the electrolysis cell operates at 5.0 V and 1.0 10 5 A, what mass of aluminum...
-
Compare Davis and Ackermann steering gears.
-
1. Assumethatlengthin Oreochromis variabilis (Victoria Tilapia) ispolygenicvaryingfrom30cmto50cm. A 30cm purebred parent is crossed with another purebred 50 cm individual and the resulting F 1...
-
1. Using appropriate examples, compare and contrast the genetic diversity of marine fish species with freshwater fish species (8 marks) 2. Your class went on a trip and discovered a crater lake on...
-
Find sin(29) given that cos(0) = and 0
-
Amazing Aquariums began as a class project on new business development. Now that the visionaries behind the idea have graduated, they want to explore their business idea and see if the concept could...
-
3. Modify the program of Example 05 so that, it takes and prints values using the following two functions respectively. void get (double *&a, int& n); void print (double *a, int n); 4. Following is a...
-
Find the coordinate vector for p relative to S = {P1, P2, P3} p = 2 - x + x2; p1 = 1 + x, p2 = 1 + x2, p3 = x + x2
-
On March 31, 2018, Gardner Corporation received authorization to issue $30,000 of 9 percent, 30-year bonds payable. The bonds pay interest on March 31 and September 30. The entire issue was dated...
-
This problem involves the design of a parallel adder-subtracter for 8-bit numbers expressed in sign and magnitude notation. The inputs X and Y are in sign and magnitude, and the output Z must be in...
-
Design a multiplier that will multiply two 16-bit signed binary integers to give a 32-bit product. Negative numbers should be represented in 2s complement form. Use the following method: First...
-
The objective of this problem is to use Verilog to describe and simulate a multiplier for signed binary numbers using Booths algorithm. Negative numbers should be represented by their 2s complement....
-
Brainstorm an example of: 1) a financial accounting issue that would be researched before a transaction is finalized 2) an accounting issue that would be researched after a transaction has been...
-
Services accounted for ____ of US imports in 2016. A) 22 percent B) 19 percent C) 16 percent D) 13 percent E) 5 percent
-
A client has established a stock option plan for its employees. It is trying to determine the amount at which the award should be recognized. Identify the location in professional standards that...

Study smarter with the SolutionInn App


