What volume of 0.955 M HCl, in milliliters, is required to titrate 2.152 g of Na 2
Question:
What volume of 0.955 M HCl, in milliliters, is required to titrate 2.152 g of Na2CO3 to the equivalence point? 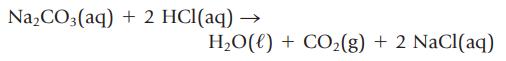
Transcribed Image Text:
Na₂CO3(aq) + 2 HCl(aq) – H₂O(l) + CO₂(g) + 2 NaCl(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
To determine the volume of 0955 M HCl required to titrate 2152 g of Na2CO3 to the equivalence po...View the full answer

Answered By

Ishrat Khan
Previously, I have worked as an accounting scholar at acemyhomework, and have been tutoring busines students in various subjects, mostly accounting. More specifically I'm very knowledgeable in accounting subjects for college and university level. I have done master in commerce specialising in accounting and finance as well as other business subjects.
5.00+
140+ Reviews
437+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
If 38.55 mL of HCl is required to titrate 2.150 g of Na 2 CO 3 according to the following equation, what is the concentration (mol/L) of the HCl solution? NaCO3(aq) + 2 HCl(aq) - 2 NaCl(aq) + CO(g) +...
-
What volume of 0.812 M HCl, in milliliters, is required to titrate 1.45 g of NaOH to the equivalence point? NaOH(aq) + HCl(aq) HO(l) + NaCl(aq)
-
You have 0.954 g of an unknown acid, H 2 A, which reacts with NaOH according to the balanced equation If 36.04 mL of 0.509 M NaOH is required to titrate the acid to the second equivalence point, what...
-
Why program planning is important in public health leadership?
-
A production manager at Ultra Clean Dishwashing Company is monitoring the quality of the companys production process. There has been concern relative to the quality of the operation in accurately...
-
A transmission tower is held by three guy wires attached to a pin at A and anchored by bolts at B, C, and D. If the tension in wire AC is 2.6kN, determine the vertical force P exerted by the tower on...
-
Consider the following exponential probability density function. a. Find P(x 6). b. Find P(x 4). c. Find P(x 6). d. Find P(4 x 6).
-
A metal rod of length 2L, diameter D, and thermal conductivity k is inserted into a perfectly insulating wall, exposing one-half of its length to an air stream that is of temperature T and provides a...
-
for the answere that are off it may be that those are the numbers for thefinal trial balance, but im not complety sure so of someone can show me the ones i got wrong and how that would be much...
-
Potassium hydrogen phthalate, KHC 8 H 4 O 4 , is used to standardize solutions of bases. The acidic anion reacts with strong bases according to the following net ionic equation: If a 0.902-g sample...
-
What volume of 0.125 M oxalic acid, H 2 C 2 O 4 , is required to react with 35.2 mL of 0.546 M NaOH? HCO4(aq) + 2 NaOH(aq) NaCO4(aq) + 2 HO(l)
-
The Marvis Company manufactures and sells a line of exclusive sportwear. The firms sales were \($650,000\) for the year just ended, and its total assets exceeded \($420,000.\) The company was started...
-
What is the formula for Bouley's coefficient of skewness?
-
What is the relation between orthocentre,circumcentre and centroid of a triangle?
-
When do we use Fourier transforms and Laplace transforms in RC/RL/RLC circuit analysis?
-
What are the protocols used in a drone?
-
How do we design a drone?
-
The following data come from the financial statements of Kawasaki, Inc., at the end of a recent year (in millions): Required 1. Prepare Kawasaki's statement of cash flows for the year ended January...
-
For the given transfer function: Vo(s) / Vi(s) = (s^2C^2R^2 + 1) / (s^2C^2R^2 + 4sCR + 1) Assumiing that 1/(CR) = 120 PI so write the matlab code to find the magnitude plot
-
Two vessels of equal volume, pressure, and temperature both containing Ar are connected by a valve. What is the change in entropy when the valve is opened, allowing mixing of the two volumes? Is S...
-
Without using equations, explain why S for a liquid or solid is dominated by the temperature dependence of S as both P and T change.
-
Solid methanol in thermal contact with the surroundings is reversibly melted at the normal melting point at a pressure of 1 atm. Are S, S surroundings , and S total positive, negative, or zero?...
-
please anwser t chart outlines are given in next two pictures if needed 1.1. Bandey, an accountant is in business with the following assals and liabilities Liabilities NP Familiar Finance NP....
-
Please help me to solve this question.
-
Question 3 2015 2014 2015 Assets Current Assets Not yet answered $45,000 Points out of 10.00 Flag question Accounts Receivable Credit Card Receivable Marketable Securities Notes Receivable Inventory...

Study smarter with the SolutionInn App


