You place 2.56 g of CaCO 3 in a beaker containing 250. mL of 0.125 M HCl.
Question:
You place 2.56 g of CaCO3 in a beaker containing 250. mL of 0.125 M HCl. When the reaction has ceased, does any calcium carbonate remain? What mass of CaCl2 can be produced?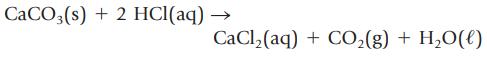
Transcribed Image Text:
CaCO3(s) + 2 HCl(aq) → CaCl₂(aq) + CO₂(g) + H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To determine if any calcium carbonate CaCO3 remains and to calculate the mass of calcium chloride Ca...View the full answer

Answered By

Madhvendra Pandey
Hi! I am Madhvendra, and I am your new friend ready to help you in the field of business, accounting, and finance. I am a College graduate in B.Com, and currently pursuing a Chartered Accountancy course (i.e equivalent to CPA in the USA). I have around 3 years of experience in the field of Financial Accounts, finance and, business studies, thereby looking forward to sharing those experiences in such a way that finds suitable solutions to your query.
Thus, please feel free to contact me regarding the same.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
You place the substance A(g) in a container. Consider the following reaction under standard conditions to produce the substance B(g): For this reaction as written, the equilibrium constant is a very...
-
In this problem you need to draw two pictures of solutions in beakers at different points in time. Time zero (t = 0) will be the hypothetical instant at which the reactants dissolve in the solution...
-
Suppose you have a subject property with a 105,000 sq. ft. lot and existing improvements for which you estimate the reproduction cost new to be $2,500,000, physical deterioration to be $400,000,...
-
A company uses exponential smoothing with trend to forecast monthly sales of its product, which show a trend pattern. At the end of week 5, the company wants to forecast sales for week 6. The trend...
-
The core of a high-temperature, gas-cooled nuclear reactor has coolant tubes of 20-mm diameter and 780-mm length. Helium enters at 600 K and exits at 1000 K when the flow rate is 8 x 10-3 kg/s per...
-
Consider the following hypothesis test. The following results are for two independent samples taken from the two populations. a. What is the value of the test statistic? b. What is the p-value? c....
-
As investment manager of Pasco Electric Companys pension plan (which is exempt from income taxes), you must choose between IBM bonds and AT&T preferred stock. The bonds have a $1,000 par value,...
-
A corporation reported net income of $2,730,000 and paid preferred cash dividends of $120,000 during the current year. There were 600,000 weighted average shares of common stock outstanding and the...
-
The cancer drug cisplatin, Pt(NH 3 ) 2 Cl 2 , can be made by reacting (NH 4 ) 2 PtCl 4 with ammonia in aqueous solution. Besides cisplatin, the other product is NH 4 Cl. (a) Write a balanced equation...
-
One half liter (500. mL) of 2.50 M HCl is mixed with 250. mL of 3.75 M HCl. Assuming the total solution volume after mixing is 750. mL, what is the concentration of hydrochloric acid in the resulting...
-
A public corporation in which you own common stock reported a WACC of 10.7% for the year in its annual report to stockholders. The common stock that you own has averaged a total return of 6% per year...
-
Consider the expression timing is everything in relation to the building of the TOMS brand. Besides the influence of recovering economic conditions and the increased affluence of potential customers,...
-
What is corporate strategy and why is it important? Choose a company with which you are familiar, and evaluate its corporate strategy, especially in regards to financial strategies. What are some...
-
Assignment Tasks: Review the following situations and for each pay period determine the employee's net pay by calculating what earnings & benefits are subject to Income Tax, Canada / Quebec Pension...
-
sample letter for signature change on bank accounts for principals of school
-
Use Excelshowing all work and formulasto complete the following: Prepare a flexible budget. Compute the sales volume variance and the variable cost volume variances based on a comparison between...
-
During December, Barnett Auction Co. completed the following transactions: Barnett's business uses the following accounts: Cash, Accounts Receivable, Supplies, Land, Accounts Payable, Notes Payable,...
-
Prove the formula for (d/dx)(cos-1x) by the same method as for (d/dx)(sin-1x).
-
The following mass spectrum is for octane. a) Which peak represents the molecular ion? b) Which peak is the base peak? c) Draw the structure of the fragment that produces the base peak. 100 80 60 40...
-
Calculate the HDI for each molecular formula. a) C 4 H 6 b) C 5 H 8 c) C 40 H 78 d) C 72 H 74 e) C 6 H 6 O 2 f) C 7 H 9 NO 2 g) C 8 H 10 N 2 O h) C 5 H 7 Cl 3 i) C 6 H 5 Br j) C 6 H 12 O 6
-
Following are the IR spectrum and mass spectrum of an unknown compound. Propose at least two possible structures for the unknown compound. 100- 80- 40- 20- 4000 3500 3000 2500 2000 1500 1000 500...
-
N.B: Please answer them ASAP , no explanation needed just the answers . Thanks in advance Ghandour Company manufactures three products of chocolate. One of the products has a net loss of $23,000 from...
-
Assume today is Sept 1, 2021. Company IMineGold is a gold mining company producing gold with plans to increase its size and valuation over the next few years. The company has invested in developing...
-
Date Activities Units Acquired at Cost Units sold at Retail Jan. 1 Beginning inventory 235 units @ $ 16.00 = $ 3,760 Jan. 10 Sales 185 units @ $ 25.00 Jan. 20 Purchase 180 units @ $ 15.00 = 2,700...

Study smarter with the SolutionInn App


