A Lewis structure for the oxalate ion is shown below. (One or more other resonance forms are
Question:
A Lewis structure for the oxalate ion is shown below. (One or more other resonance forms are also possible.)
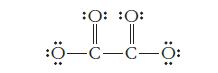
What is the correct charge on the oxalate ion? What type of orbital hybridization is expected for each of the carbon atoms in this structure? How many sigma bonds and how many pi bonds does the structure contain?
Transcribed Image Text:
-C-C- :O: :0:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Based on the valence of each atom a neutral molecule ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The azide ion, N3-, is linear with two N-N bonds of equal length, 1.16 Ã. (a) Draw a Lewis structure for the azide ion. (b) With reference to Table 8.5, is the observed bond length consistent...
-
Methyl cyanoacrylate, C 5 H 5 NO 2 , is the compound commonly sold as super glue. The glue works through a polymerization reaction, in which molecules of methyl cyanoacrylate form strong chemical...
-
The lactic acid molecule, CH3CH (OH) COOH, gives sour milk its unpleasant, sour taste. (a) Draw the Lewis structure for the molecule, assuming that carbon always forms four bonds in its stable...
-
The mass of the crane?s boom is 9000 kg. Its weight acts at?G. The sum of the moments about?P?due to the boom?s weight, the force exerted at?B?by the cable?AB,?and the force exerted at?C?by the...
-
Real options can be analyzed using a scenario approach with decision trees or using the Black-Scholes Option Pricing Model. What are the pros and cons of the two approaches? Is one procedure better...
-
List the aims of both the Economic Community of West African States and the African Union.
-
Does the contractor have a good working relationship with the architect, material suppliers, electricians, plumbers, carpenters, and other personnel needed for the project?
-
Baker, CPA, was engaged to audit Mill Company's financial statements for the year ended September 30, 20X1. After obtaining an understanding of Mill's internal control structure, Baker decided to...
-
In 150 words!! Please help.. 5. Karla markets a bell she claims will automatically quiet a crying baby. She advertises on television that the bell has a certain tone babies love and shows a baby...
-
Nearly all of the other elements form binary compounds with hydrogen. Based on the electronegativity values shown in Figure 7.7, with what category of elements will hydrogen form bonds in which the...
-
The following molecules have similar formulas, but each has a different shape. Draw Lewis structures for these compounds and then determine their molecular shapes. (a) SiF 4 , (b) KrF 4 , (c) SeF 4
-
A ball is dropped from a height of 1 m and loses 10 percent of its kinetic energy when it bounces on the ground. To what height does it then rise?
-
1. Define Image? 2. What is Dynamic Range? 3. Define Brightness? 4. What do you mean by Gray level? 5. What do you mean by Color model? 7. List the hardware oriented color models 8. What is Hue and...
-
11. Define Resolutions 12. What is meant by pixel? 13. Define Digital image 14. What are the steps involved in DIP? 15. What is recognition and Interpretation?
-
16. Specify the elements of DIP system 17. List the categories of digital storage 18. What are the types of light receptors? 19. Differentiate photopic and scotopic vision Photopic vision Scotopic...
-
21. Define subjective brightness and brightness adaptation 22. Define weber ratio 23. What is meant by machband effect? Machband effect means the intensity of the stripes is constant. Therefore it...
-
26. Define sampling and quantization 27. Find the number of bits required to store a 256 X 256 image with 32 gray levels 28. Write the expression to find the number of bits to store a digital image?...
-
Give a specific example in which the variant system of CAPP is desirable and an example in which the generative system is desirable.
-
On October 31 Juanita Ortega, owner of Outback Guide Service, received a bank statement dated October 30. Juanita found the following: 1. The checkbook has a balance of $2,551.34. 2. The bank...
-
Show using an example that the following two formulations of the Pauli exclusion principle are equivalent: a. Wave functions describing a many-electron system must change sign under the exchange of...
-
Calculate the terms that can arise from the configuration np 1 np 1 , n n. Compare your results with those derived in the text for np 2 . Which configuration has more terms and why?
-
Calculate the terms that can arise from the configuration np 1 np 1 , n n. Compare your results with those derived in the text for np 2 . Which configuration has more terms and why?
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...
-
You would like to have a balance of $600,000 at the end of 15 years from monthly savings of $900. If your returns are compounded monthly, what is the APR you need to meet your goal?
-
Explain the importance of covariance and correlation between assets and understanding the expected value, variance, and standard deviation of a random variable and of returns on a portfolio.

Study smarter with the SolutionInn App


