Methyl cyanoacrylate, C 5 H 5 NO 2 , is the compound commonly sold as super glue.
Question:
Methyl cyanoacrylate, C5H5NO2, is the compound commonly sold as super glue. The glue works through a polymerization reaction, in which molecules of methyl cyanoacrylate form strong chemical bonds to one another and to the surfaces being glued. This process is initiated by traces of water on the surfaces. The skeletal arrangement of the atoms in methyl cyanoacrylate is shown below. Complete the Lewis structure of the compound by adding lone pairs of electrons or multiple bonds where appropriate.
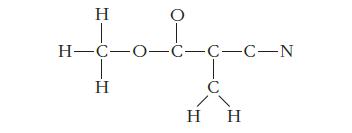
Using your Lewis structure, predict the hybridization for the six central atoms (the five carbon atoms and the leftmost oxygen atom). How many sigma bonds and how many pi bonds does your structure contain?
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





