Chromium can be detected in atomic absorption spectroscopy by monitoring the absorbance of UV light at a
Question:
Chromium can be detected in atomic absorption spectroscopy by monitoring the absorbance of UV light at a wavelength of 357.8 nm. What is the energy of a photon of this light?
Strategy We know the connection between photon energy and wavelength, which is given by Equation 6.3. Again, care with units requires conversion from nanometers to meters.
Equation 6.3.
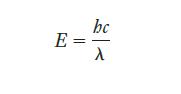
Transcribed Image Text:
E = bc λ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
Analyze Your Answer The result is a very small number But we should realize that P...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
A helium-neon laser emits laser light at a wavelength of 632.8 nm and a power of 2.3 mW. At what rate are photons emitted by this device?
-
The laser used in cornea surgery to treat corneal disease is the excimer laser, which emits ultraviolet light at a wavelength of 193 nm in air. The index of refraction of the cornea is 1.376. What...
-
The active medium in a particular laser that generates laser light at a wavelength of 694 nm is 6.00 cm long and 1.00 cm in diameter. (a) Treat the medium as an optical resonance cavity analogous to...
-
Tin - Can, Inc. Aircraft ( TCAI ) R&D Project Management Problem Your group is hired to help TCAI Project Manager to solve the following problem. Using the activity time estimates and activity...
-
Thome Company uses a flexible budget for manufacturing overhead based on direct labor hours. Variable manufacturing overhead costs per direct labor hour are as follows. Indirect labor ........ $1.00...
-
What is a moral dilemma?
-
15. The price of a non-dividend-paying stock is $100 and the continuously compounded risk-free rate is 5%. A 1-year European call option with a strike price of $100 e0.051= $105.127 has a premium of...
-
The following information is available for Laurel Company, a wholesale company: Expected sales volume: October . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ....
-
please help asap 2. The income statements for urban outfits, Ina are presented below. urban outfits inc Income statements Year ended December 31 current year Sacs revenue $875,059 cost of goods sold...
-
Unlike XRF, AAS cannot be used for nondestructive testing. Explain why not.
-
Consider a room that is 14 ft 20 ft with an 8-ft ceiling. (a) How many molecules of air are present in this room at 20C and 750 torr? (b) If a pollutant is present at 2.3 ppm, how many pollutant...
-
What is the pressure on a horizontal surface with an area of 2 m 2 that is 4 m underwater?
-
7. Chicago Corp. obtained the following information from the Raw Materials Inventory account and purchasing records for the first quarter of the current year: Beginning Raw Materials Ending Raw...
-
Suppose that i t =6% (n=1), and that future short term interest rates (n=1) for the next 3 years (starting next year) are expected to be: 4%, 2%, 2%. Suppose that the liquidity premium is zero for...
-
Mechanical Vibrations HW Use the modal analysis and numerical integration to compute and plot the time response of the system, which has the equations of motion [8 0 01 (1) 48 -12 01(x1 0 0 8 02-12...
-
Submit excel file with graph and exchange rate analysis. FOREIGN EXCHANGE RATESTHE YEN FOR DOLLARS. The Federal Reserve System Web site, www.federalreserve.gov/releases/H10/hist , provides historical...
-
Part 1: There are many types of communication styles used in the workplace. Choose what you think is your leadership style: north, south, east, or west. Click The Leadership Compass Self-Assessment...
-
Millbrook Golf Club, Inc., provides the following data for the year ended June 30, 20X9. Prepare the operating activities section of Millbrook Golf Club, Inc.'s, statement of cash flows for the year...
-
What is the back work ratio? What are typical back work ratio values for gas-turbine engines?
-
Compound A has molecular formula C 9 H 8 O 2 and exhibits a strong signal at 1740 cm -1 in its IR spectrum. Treatment with two equivalents of LAH followed by water gives the following diol. Identify...
-
Rank each set of compounds in order of increasing acidity: a. b.
-
Malonic acid has two acidic protons: The pKa of the first proton (pK 1 ) is measured to be 2.8, while the pK a of the second proton (pK 2 ) is measured to be 5.7. (a) Explain why the first proton is...
-
Suppose First Fidelity Bank engaged in the following transactions: (Click the icon to view the transactions.) Journalize the 2018 and 2019 transactions on First Fidelity's books. Explanations are not...
-
Financial data for Joel de Paris, Inc., for last year follow: Joel de Paris, Inc. Balance Sheet Beginning Balance Ending Balance Assets Cash Accounts receivable Inventory Plant and equipment, net...
-
Supply costs at Coulthard Corporation's chain of gyms are listed below: March April May June July August September October November Client-Visits 11,666 11,462 11,994 13,900 11,726 11, 212 12,006...

Study smarter with the SolutionInn App


