MTBE, C 5 H 12 O, is one of the additives that replaced tetraethyl-lead in gasoline. (See
Question:
MTBE, C5H12O, is one of the additives that replaced tetraethyl-lead in gasoline. (See Example Problem 4.6 and Section 4.6.) How many moles of O2 are needed for the complete combustion of 1.50 mol of MTBE?
Example Problem 4.6
MTBE (methyl tert-butyl ether) has been used as an additive in gasoline. The compound is produced by reacting methanol and isobutene, according to the following equation:
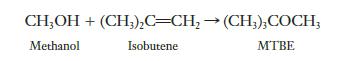
If 45.0 kg of methanol is allowed to react with 70.0 kg of isobutene, what is the maximum mass of MTBE that can be obtained?
Strategy We are given amounts of two different reactants (methanol and isobutene). So we should realize this is a potential limiting reactant situation. We can identify the limiting reactant as in the previous examples: Choose one reactant and find the amount of the other that would be needed to react with it. Comparing that result with the given amount, we can determine which of the reactants is limiting. Once we have done that, it will be straightforward to calculate the expected amount of product.
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





