Referring to Figure 6.15, draw a 4p orbital, showing all of its nodes. Figure 6.15 The 3s
Question:
Referring to Figure 6.15, draw a 4p orbital, showing all of its nodes.
Figure 6.15
Transcribed Image Text:
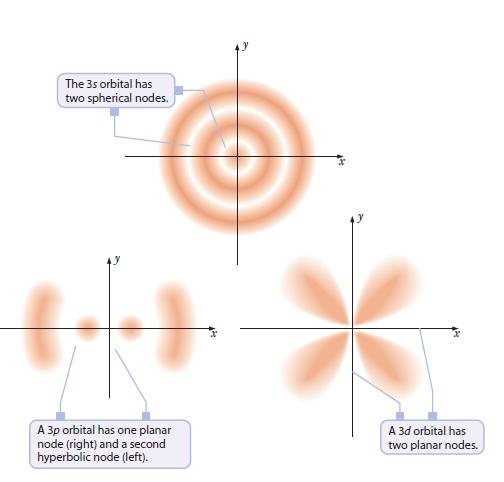
The 3s orbital has two spherical nodes. + A 3p orbital has one planar node (right) and a second hyperbolic node (left). A 3d orbital has two planar nodes.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
The illustration of t...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Determine the force in each member of the space truss in E9.3.27 if the magnitudes of F and F are 8 kip and 4 kip, respectively. State whether each member is in tension or compression. 2 ft F2 2 ft...
-
A certain shoe conies in 5 different styles with each style available in 4 distinct colors. If the store wishes to display pairs of these shoes showing all of its various styles and colors, how many...
-
The first payroll in October covered the two workweeks that ended on September 26 and October 3. This payroll transaction has been entered for you in the payroll register, the employees' earnings...
-
Discuss the differences between @classmethod , @staticmethod , and instance methods in Python.
-
The Dinkle and Frizell Dental Clinic provides both preventive and orthodontic dental services. The two owners, Reese Dinkle and Anita Frizell, operate the clinic as two separate investment centers:...
-
Delta Oil Company uses the successful-efforts method to account for oil exploration costs. Delta started business in 2014 and prepared the following income statements: The company choose to change to...
-
18. Using the information in Table 9, verify that it is possible to derive the 8-quarter dollar interest swap rate from the 8-quarter euro interest swap rate by using equation (13).
-
Why is it important for the functional areas to be involved in the project from the time of the original proposal?
-
Title: Analysis of Capital Reconstruction: Need and Impact on Financial Position of a Selected Company Objective The objective of this project is to analyze the need for capital reconstruction and...
-
Define the term nodal plane (or node).
-
Tungsten alloys are often used for parts that must withstand high temperatures. Use the periodic table to determine the electron configuration of tungsten (W). Strategy Begin by finding tungsten in...
-
When a woman is randomly selected and measured for blood pressure, the systolic blood pressure is found to be 61 mm Hg. Determine whether the given values are from a discrete or continuous data set.
-
Contract for construction crew and equipment 8 Build parking lots Exterior lighting 11 7 20 12 Build foundation Start Interior Interior 12 9 electrical Final wiring finish Purchase 8 14 12 material...
-
Mad Hatter Enterprises purchased new equipment for $369,000, terms f.o.b. shipping point. Other costs connected with the purchase were as follows: State sales tax Freight costs Insurance while in...
-
Write down a C program that takes runs scored by a batsman and prints the status according to the following policy: Runs scored >80 50-79 30-49 10-29 <10 Grade Excellent 4 Very Good Good Average Poor
-
Consider the standard two-period maximization problem for investor j over s states of nature: Subject to S max u(c) + (s)u(c;}(s)) S=1 Cjo + q(s) C; (s) = Wjo +244) S=1 where all terms are as defined...
-
At what point should a leader cease gathering data, take the risk, and simply make the decision? Support your position.
-
The accounting records of Jasmine Pharmaceuticals, Inc., reveal the following: Required Compute cash flows from operating activities by the direct method. Use the format of the operating activities...
-
Research corporate acquisitions using Web resources and then answer the following questions: Why do firms purchase other corporations? Do firms pay too much for the acquired corporation? Why do so...
-
Identify the number of steps (patterns) for the mechanisms in Problems. For example, the patterns for the first two are: a: ±H ± LG Nuc attack This mechanism exhibits a proton transfer...
-
Consider the structure of the compound below. (a) Identify each position where an S N 2 reaction is likely to occur. (b) Identify each position where an S N 1 reaction is likely to occur. Cl CI TsO....
-
Does each of the following solvents favor an SN2 reaction or an SN1 reaction? a) b) c) d) (e) MeOH (f ) CH 3 CN (g) HMPA (h) NH 3 %24
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...
-
In the context of portfolio theory, what is diversification primarily intended to do ? A ) Increase returns. B ) Reduce risk. C ) Maximize tax efficiency. D ) Simplify investment management.

Study smarter with the SolutionInn App


