Sulfur dioxide can be generated by the reaction of sodium hydrogen sulfite with hydrochloric acid. (a) Write
Question:
Sulfur dioxide can be generated by the reaction of sodium hydrogen sulfite with hydrochloric acid.
(a) Write the balanced chemical equation for the reaction, given that the other products are sodium chloride and water.
(b) If 1.9 g of sodium hydrogen sulfite is reacted with excess HCl, what mass of SO2 is produced?
(c) If the gas generated is in a 100-mL vessel at 25°C, and 0.05 mol of O2 is introduced, equilibrium between SO2, O2, and SO3 will be established.
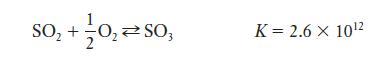
Calculate the equilibrium concentration of each species.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





