The following table presents the abundances and masses of the isotopes of zinc. What is the atomic
Question:
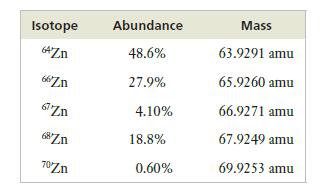
The following table presents the abundances and masses of the isotopes of zinc. What is the atomic weight of zinc?
Transcribed Image Text:
Isotope 64Zn 66Zn 67 Zn 68Zn 70Zn Abundance 48.6% 27.9% 4.10% 18.8% 0.60% Mass 63.9291 amu 65.9260 amu 66.9271 amu 67.9249 amu 69.9253 amu
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate the atomic weight of zinc we need to use the ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
(A) The masses and percent isotopic abundances of the three naturally occurring isotopes of silicon are 28 Si, 27.9769265325 u, 92.223%; 29 Si, 28.976494700 u, 4.685%; 30 Si, 29.973377017 u, 3.092%....
-
Europium has two stable isotopes, 151 Eu and 153 Eu, with masses of 150.9197 u and 152.9212 u, respectively. Calculate the percent abundances of these isotopes of europium. EXAMPLE 2.2 Calculating...
-
Gallium has two naturally occurring isotopes, 69 Ga and 71 Ga, with masses of 68.9257 u and 70.9249 u, respectively. Calculate the percent abundances of these isotopes of gallium. EXAMPLE 2.2...
-
Lamonda Corp. uses a job order cost system. On April 1, the accounts had the following balances: The following transactions occurred during April: (a) Purchased materials on account at a cost of...
-
Which amortization method is required for intangibles? Are there any exceptions?
-
You have found the following historical information for the Daniela Company over the past four years: Earnings are expected to grow at 11 percent for the next year. Using the companys historical...
-
On 1st April, 2005, Star Paper Limited purchased a plant for Rs. 1,50,000 and paid Rs. 10,000 as freight on its carriage. Depreciation was provided at 10% per annum on the written down value method...
-
At the end of 2015, Carpenter Co. has accounts receivable of $700,000 and an allowance for doubtful accounts of $54,000. On January 24, 2016, the company learns that its receivable from Megan Gray is...
-
what are types of organistionsal culture
-
The atomic weight of copper is 63.55 amu. There are only two isotopes of copper, 63 Cu with a mass of 62.93 amu and 65 Cu with a mass of 64.93 amu. What is the percentage abundance of each of these...
-
Mercury is 16.716 times more massive than 12 C. What is the atomic weight of mercury? Remember to express your answer with the correct number of significant figures.
-
Solve each equation or inequality. Give the solution set in set notation for equations and in interval notation for inequalities. 5x (3+x) 2(3x + 1) -
-
The hip roof shown in the below Figure 2 is constructed of 2x10 rafters spaced 16 inches on center. The hip rafters are 1 -inch-wide by 12-inch-high GLBs. The roof has a slope of 4:12. Prepare a list...
-
2. Estimate the populations of Fargo, ND and Bismarck, ND in years of 2040 and 2050. Select a single value of population that you would use for design purposes in each year. You need to specify and...
-
A liquid mixture of 65 mole% n-nonane and 35 mol% n-octane enters a flash unit. In the flash unit, the pressure is reduced to 1 atm and half of the liquid is evaporated. find the temperature in the...
-
To gain a deep understanding of SAPPI LIMITED's industry and competitive environment, answer the following questions before the company can embark on a "new strategy" breakaway. Does this industry...
-
What communication tools can a manager use to construct and deliver constructive and timely feedback to their employees? Discuss the various communication tools (i.e. email, phone, text, social...
-
Convert the following to units in which c = 1, expressing everything in terms of m and kg: (a) Worked example: 10 J. In SI units, 10 J = 10 kgm2 s2. Since c = 1, we have 1 s = 3 108 m, and so 1 s2 =...
-
(a) Prove that form an orthonormal basis for R3 for the usual dot product. (b) Find the coordinates of v = (1, 1, 1)T relative to this basis. (c) Verify formula (5.5) in this particular case. 48-65...
-
Determine the configuration for every chirality center in each of the following compounds. a. b. c. HO H- - OH H,OH
-
Suggest an efficient synthesis for each of the following transformations: a. b.
-
Using acetylene as your only source of carbon atoms, identify a synthetic route for the production of 2-bromobutane.
-
he median home price in Arlington, Virginia, is $634,000. If the assessment rate is 100%, what is the assessed value
-
In the spot market, 1 U.S. dollar can be exchanged for 100 Japanese yen. In the 1-year forward market, 1 U.S. dollar can be exchanged for 115 Japanese yen. The 1-year, risk-free rate of interest is 3...
-
An extract from the financial records of Alpha plc as at 3 1 December 2 0 2 1 is below: 0 0 0 0 0 0 Bank 1 4 0 Electricity ( for the workshop ) 3 3 0 Chairman s salary 5 0 0 Insurance 1 7 0 Machinery...

Study smarter with the SolutionInn App


