Use the standard reduction potentials for the reactions: to calculate the value of Ksp for silver chloride
Question:
Use the standard reduction potentials for the reactions:
![]()
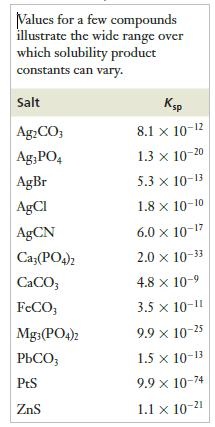
to calculate the value of Ksp for silver chloride at 298 K. How does your answer compare with the value listed in Table 12.4?
Table 12.4
Transcribed Image Text:
AgCl(s) +eAg(s) + Cl (aq) and Ag+ (aq) + e→Ag(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
K sp 17 10 10 T...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Use the standard reduction potentials to find the equilibrium constant for each of the following reactions at 25°C: (a) (b) (c) Br2(1) + 21-(aq )- 2Br_(aq) + 12(s) 5Fe2 + (aq) +MnO4 (aq ) + 8H +...
-
The standard reduction potentials of the following half-reactions are given in Appendix E: (a) Determine which combination of these half-cell reactions leads to the cell reaction with the largest...
-
Using the standard reduction potentials listed in Appendix E, calculate the equilibrium constant for each of the following reactions at 298 K: (a) Cu(s) + 2 Ag+ (aq) Cu2+ (aq) + 2 Ag(s) (b) 3 Ce4+...
-
MOSS COMPANY Income Statement \ table [ [ , ] , [ Sales For Year Ended December 3 1 , 2 0 2 1 , $ 5 2 5 , 0 0 0 es MOSS COMPANY Income Statement For Year Ended December 31, 2021 Sales Cost of goods...
-
List five or more topics that an organization might feature in a press release.
-
In 1991, Social Security and Medicare taxes were itemized separately on paycheck stubs and tax forms for the first time. The table on the right gives a historical look at Social Security and Medicare...
-
Explain the stages in the personal selling process.
-
Dali Corporation??s comparative balance sheet for current assets and liabilities was as follows: Adjust net income of $240,000 for changes in operating assets and liabilities to arrive at net cash...
-
Tony and Suzie graduate from college in May 2021 and begin developing their new business. They begin by offering clinics for basic outdoor activities such as mountain biking or kayaking. Upon...
-
Calculate the equilibrium constant for the following reactions using data from the standard reduction potential tables. (a) Cl(g) + 2 Br (aq) Br(g) + 2 Cl(aq) (b) Ni(s) + 2 Ag+ (aq) 2 Ag(s) + Ni+...
-
The equilibrium constant for a reaction is 3 10 15 . (a) Without carrying out any calculation, discuss whether G for the reaction is positive or negative. (b) Calculate G for this reaction.
-
P5-7 (Preparing adjusting journal entries)
-
Write down at leastfive items (durable goods, not food) that you purchase and their sourcing (where each is from). For example, a shirt may be assembled in China, designed in the US, and made from...
-
I agree that overtime can be tricky in different countries. As we've seen with piecework, it is hard to implement it in countries where people are not motivated to work past their regular hours, even...
-
time (in seconds). Find a formula for 1, if V = 5t(t Suppose that an object's acceleration function is given by a = 4t+ 6. The object's initial velocity is 4, and the initi position is 9. Find the...
-
Determine the specific major and foundational managerial discoveries and findings from each era as most pivotal for management evolution (Early Management Era, Social Management Era, Scientific...
-
Identify the three major pricing strategies and discuss the important key factors that impact setting prices. Explain what time of pricing strategy your assigned brand uses and why you believe this...
-
Why do you think that the triage system is effective in controlling operations in Accident and Emergency departments?
-
In a certain school district, 3% of the faculty use none of their sick days in a school year. Find the probability that 5 faculty members selected at random used no sick days in a given year.
-
The truss is made of three A-36 steel members, each having a cross-sectional area of 400 mm 2 . Determine the horizontal displacement of the roller at C when P = 8 kN. 5 kN 0.8m 0.8 m. 0,6 m-
-
The A-36 steel drill shaft of an oil well extends 12 000 ft into the ground. Assuming that the pipe used to drill the well is suspended freely from the derrick at A, determine the maximum average...
-
The 2014-T6 aluminium rod has a diameter of 30 mm and supports the load shown. Determine the displacement of end A with respect to end E. Neglect the size of the couplings. 8 kN 2 kN 6 kN 4 kNY -2m-...
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...
-
Sam owns a 25% in Spade, LLC. In 2021, Spade reports $100,000 or ordinary income. What is Sams qualified business income (QBI) deduction? answer is 5,000 but please show how to get it
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...

Study smarter with the SolutionInn App


