What type of hybridization would be expected for the central atom X in each of the following
Question:
What type of hybridization would be expected for the central atom X in each of the following Lewis structures?
(a) 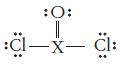
(b) ![]()
(c) 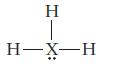
Transcribed Image Text:
:O: :Cl—x— Cl: -X-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a sp C1 ...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Solve the right triangle. Round decimal answers to the nearest tenth. A 11 B 5 C
-
What hybrid orbitals would be expected for the central atom in each of the following molecules or ions? a. SeCl2 b. NO2 c. CO2 d. COF2
-
What hybrid orbitals would be expected for the central atom in each of the following molecules or ions? a. GeCl4 b. PBr3 c. BeF2 d. SO2
-
Consider the no-trade input/output situation presented in the following table for countries X and Y. Assuming that free trade is allowed, develop a scenario that will benefit the citizens of...
-
(1) Draw NPV profiles for Franchises L and S. At what discount rate do the profiles cross? (2) Look at your NPV profile graph without referring to the actual NPVs and IRRs. Which franchise or...
-
Four couples at a dinner party play a board game after the meal. They decide to play as teams of two and to select the teams randomly. All eight people write their names on slips of paper. The slips...
-
If they obtained a mortgage with a very small down payment, what concerns might Zoe and Luis encounter?
-
The following information for Lesky Corporation covers the year ended December 31, 2010: Required Change this statement to a multiple-step format, as illustrated in this chapter. LESKY CORPORATION...
-
what is the frims profit margin
-
The number of hybrid orbitals formed must always be equal to the number of atomic orbitals combined. Suggest a reason why this must be true.
-
What observation about molecules compels us to consider the hybridization of atomic orbitals?
-
Specialty Light Bulbs management anticipates selling 3,000 light bulbs this year at a price of $15 per bulb. It costs Specialty $10 in variable costs to produce each light bulb, and the fixed costs...
-
Evaluation a. Evaluate the effectiveness of social media marketing campaign for instagram, facebook and pintrest ?based on your KPIs for example account reached, content reached, likes, shares,...
-
A study was performed at a university to analyze whether the preference for hamburgers or fried chicken is related to the gender of the student. This table lists the results of the study. At a =...
-
A 20-lb homogeneous box has tipped and is resting against a 40-lb homogeneous box as shown in figure attached. The coefficient of friction between box A and the floor is 0.7, and between box B and...
-
The Taylor series for natural logarithm (with base e) In(1+r) is In(1+2) -(-1)+1 for <1. (a) Write a user-defined function using loop that determines In(1+x) using the above Taylor series. Your...
-
Question 1: [up to 4 pts] Suppose that a = 1, a2 = 2, a3 = = 3, and an = an-3 for all n 4. If an integral with respect to y is used to find the area of R, what should the upper limit of integration...
-
List and explain the advantages of computer-aided NC programming?
-
The population of Detroit, Michigan, decreased from 1,027,974 in 1990 to 688,701 in 2013 (Source: U.S. Census Bureau). Find the average rate of change in the population of Detroit, Michigan, over the...
-
Using Figures 23.9 and 23.10, explain why Î Ï 2 g < 0 and ÎÏ 2 u > 0 outside of the bonding region of H + 2 . Figure 23.9 Figure 23.10 Bonding - Antibonding . , Bonding...
-
Using Figures 23.9 and 23.10, explain why 2 g < 0 and 2 u > 0 outside of the bonding region of H + 2 . Figure 23.9 Figure 23.10 Bonding Antibonding H.
-
Consider the molecular electrostatic potential map for the BeH 2 molecule shown here. Is the hydrogen atom (shown as a white sphere) an electron acceptor or an electron donor in this molecule?
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


