A gas sample in a 1.2-L container holds 0.22 mol N 2 and 0.13 mol O 2
Question:
A gas sample in a 1.2-L container holds 0.22 mol N2 and 0.13 mol O2. Calculate the partial pressure of each gas and the total pressure at 50 °C.
Strategy
Use the ideal gas law to calculate the partial pressure of each gas in the container, and sum these two numbers to obtain the total pressure.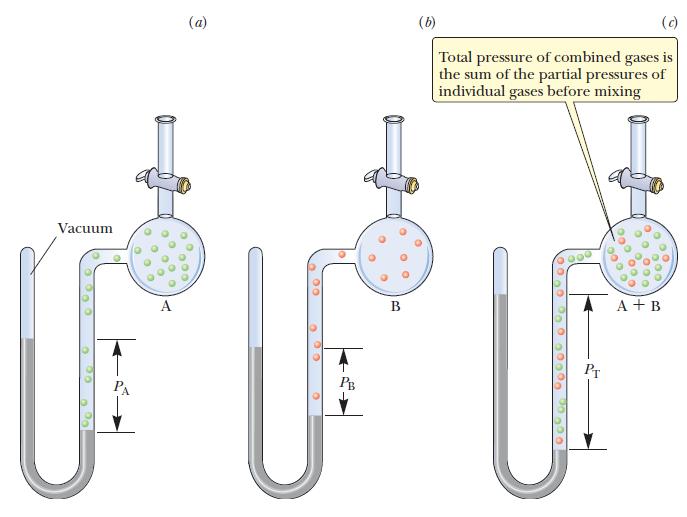
Transcribed Image Text:
Vacuum (a) 200 PB 0 0 D 00 B (b) O (c) Total pressure of combined gases is the sum of the partial pressures of individual gases before mixing 0000 0000 ooo! 0000 00 O 0.000 30 000 A + B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
Make a table of the information given PN Po PN Poz VN2 12 L Vo 12 L ...View the full answer

Answered By

Ankur Gupta
I have a degree in finance from a well-renowned university and I have been working in the financial industry for over 10 years now. I have a lot of experience in financial management, and I have been teaching financial management courses at the university level for the past 5 years. I am extremely passionate about helping students learn and understand financial management, and I firmly believe that I have the necessary skills and knowledge to effectively tutor students in this subject.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A 12.5-L scuba diving tank contains a heliumoxygen (heliox) mixture made up of 24.2 g of He and 4.32 g of O 2 at 298 K. Calculate the mole fraction and partial pressure of each component in the...
-
Alset Inc. is considering manufacturing and selling high-end electric automobiles for the next four years. It hired two research and development teams. Team #1: This team believes that it would be...
-
A mixture containing 0.765 mol He (g), 0.330 mol Ne (g) and 0.110 mol Ar (g) is confined in a 10.00-L vessel at 25oC. (a) Calculate the partial pressure of each of the gases in the mixture. (b)...
-
Watts and Strogatz proposed a hybrid model that contains typical links of vertices near each other (people know their geographic neighbors), plus some random long-range connection links. Plot the...
-
The classical probability distribution function for a particle in a box of length L is given by P(x) = 1/L. Use this to find (x) and (x2)for a classical particle in such a box.
-
A camera is being used with a correct exposure at f/4 and a shutter speed of (1/16) s. In order to photograph a rapidly moving subject, the shutter speed is changed to (1/128) s. Find the new f...
-
Construct and interpret sensitivity, overlay, trend, and box-whisker charts for a simulation model.
-
On January 1, 2013, Allied Industries leased a high-performance conveyer to Karrier Company for a four-year period ending December 31, 2016, at which time possession of the leased asset will revert...
-
The reason for the REDFIN timing of the IPO? The length of time between registration and going to market, and Why did it take so long? Economic factors impacting the industry after the Redfin IPO?...
-
Find point estimates for the mean and standard deviation of the Months Customer data in the Credit Risk Data file. Draw five random samples of sizes 50 and 250 from the data using the Sampling tool....
-
Why do 1 mol N 2 and 1 mol O 2 both exert the same pressure if placed in the same 20-L container? Is the mass of the gas sample the same in both cases? Explain why it is the same or diff erent, and...
-
Hydrogen, H 2 , and chlorine, Cl 2 , react to form hydrogen chloride, HCl. Calculate the volume of HCl formed by the reaction of 2.34 L H 2 and 3.22 L Cl 2 .
-
What was Bank Transfer Day?
-
Identify at least two business systems that support the development of effective work relationships Briefly explain how each system supports the development of effective work relationships.
-
Power and Influence Personal Plan - How will you navigate the realms of power and influence? Why is this personal plan important for you? What do you want to achieve? do a table with SMART goals -...
-
A single-stage trickling-filter plant is proposed for treating a dilute wastewater with a BOD concentration of 170 mg/L. The plant is located in a warm climate, and the minimum wastewater temperature...
-
For the first assignment for this course, compose a written document that contains the following: A description and assessment of your past experiences with policy and program planning, either your...
-
What are the key motivators driving consumer purchasing decisions in our industry? How do consumers perceive our brand compared to competitors, and what factors influence brand loyalty?
-
A ceramic part for a jet engine has a yield strength of 75,000 psi and a plane strain fracture toughness of 5,000 psi(in. To be sure that the part does not fail, we plan to ensure that the maximum...
-
Explain how the graph of each function can be obtained from the graph of y = 1/x or y = 1/x 2 . Then graph f and give the (a) Domain (b) Range. Determine the largest open intervals of the domain over...
-
Would the trial wave function have been a suitable choice for the calculations carried out in Section 21.4? Justify your answer. 3) 48) () %3D 0 < x < a
-
Is (1, 2) = 1s(1) (1)1s(2) (2) + 1s(2) (2)1s(1) (1) an eigenfunction of the operator S z ? If so, what is its eigenvalue M S ?
-
Calculate the angles that a spin angular momentum vector for an individual electron can make with the z axis.
-
A company is evaluating a new 4-year project. The equipment necessary for the project will cost $3,300,000 and can be sold for $650,000 at the end of the project. The asset is in the 5-year MACRS...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
I need to see where the calculations for this problem come from plz. 5. Award: 4.00 points Lucido Products markets two computer games: Claimjumper and Makeover. A contribution format income statement...

Study smarter with the SolutionInn App


