A mixture of carbon monoxide and oxygen gas reacts as shown below. (a) Write the balanced
Question:
A mixture of carbon monoxide and oxygen gas reacts as shown below.
(a) Write the balanced equation (remember to express the coefficients as the lowest set of whole numbers).
(b) Name the product.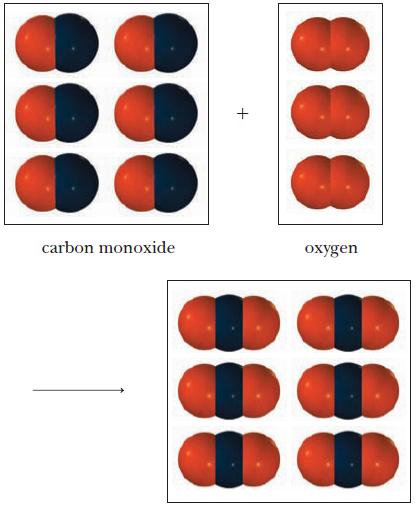
Transcribed Image Text:
carbon monoxide + 888 oxygen
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
a 2CO ...View the full answer

Answered By

Aun Ali
I am an Associate Member of Cost and Management Accountants of Pakistan with vast experience in the field of accounting and finance, including more than 17 years of teaching experience at university level. I have been teaching at both undergraduate and post graduate levels. My area of specialization is cost and management accounting but I have taught various subjects related to accounting and finance.
5.00+
13+ Reviews
32+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A mixture of sulfur dioxide and oxygen gas reacts as shown below. (a) Write the balanced equation (remember to express the coefficients as the lowest set of whole numbers). (b) Name the product....
-
Water gas, a mixture of carbon monoxide and hydrogen, is produced by treating carbon (in the form of coke or coal) with steam at high temperatures. (See Study Question 83. Not all of the carbon...
-
1. (12 points) Consider the state diagram of the DFA M in Figure 1 and answer the following questions. 0 0 0,1 0 1 91 92 93 94 1 0,1 95 Figure 1: A DFA M for Question 1. (a) Give the formal...
-
Explain why placing soy sauce in airtight bottles was more successful for long-distance shipping than simply placing the sauce in barrels.
-
A 50- coaxial cable feeds a 75 + j20- dipole antenna. Find T and s.
-
3. (6 pts) You initiated a transaction to purchase a 2.875% coupon 10-year U.S. Treasury Note on Wednesday 9/6/2018. The maturity date of the note is 5/15/2026 and its yield to maturity is 2.950%....
-
Variance analysis involves ------------- and ------------- of variances.
-
Suppose the production possibility frontier for cheeseburgers (C) and milkshakes (M) is given by C + 2M = 600 a. Graph this function. b. Assuming that people prefer to eat two cheeseburgers with...
-
A firm that manufactures telecommunication equipment. It reported earnings before interest and taxes of $ 400 million in the most recent financial year, and faced a 40% tax rate. In addition, the...
-
Selected transactions of Coromandel Ltd are given on the next page. The company uses straight-line depreciation and calculates depreciation expense to the nearest whole month. 2015 Jan. 4 April 10...
-
Given the following equation, calculate the mass (in grams) of AlCl 3 that can be produced from 4.40 g Al and 12.0 g Cl 2 ? 2A1+3C1 2A1C13
-
Describe what is meant by the statement, In a combustion reaction, C 2 H 4 is the limiting reactant and oxygen is present in excess.
-
The put premium per British pound on March 1 is \($0.04,\) the settlement date is September 19, and the strike price is \($1.80.\) A speculator anticipates that the spot rate for the pound will fall...
-
Explain the way to generate value of business for business sustainable development with one example (5 points) Identify and explain the key points for positive results for business sustainable...
-
Systematization is the most common way of causing specific practices and ways of behaving to turn out to be solidly settled inside an association or society. It includes making clear principles,...
-
Imagine that you are working at Seneca Bank as a Financial Planner. You are working hard to establish your Financial Planning practice and you realize there are many aspects involved in successfully...
-
Consider all of the analytical tools that Marr has presented. Choose 3 and research ways that specific businesses (after 2022 and other than those that Marr has mentioned) have used each of the...
-
First, tell us about an organization that you thoroughly enjoyed working for and explain what the dynamics of that culture was like. Please go into detail. Second, tell us about an organization that...
-
(a) In most peptides, the amide bonds have the Z conformation; explain why. (b) One particular amino acid residue in the PepC position adopts the E conformation in some cases. Which amino acid...
-
Teasdale Inc. manufactures and sells commercial and residential security equipment. The comparative unclassified balance sheets for December 31, 2015 and 2014 are provided below. Selected missing...
-
In each case, identify the most likely position at which monobromination would occur. (a) (b) (c) (d) N.
-
Identify the carboxylic acid and the alcohol that are necessary in order to make each of the following compounds via a Fischer esterification: a. b. c. CH 3 CH 2 CO 2 C (CH 3 ) 3
-
Determine the structures of compounds A through F: Na,Cr,0, H2SO4, H20 [H'] A EtO soch 1) LIAI(OR)H `2) H20 xS NH3
-
Aecerty 1067687 was completed with the folowing charaderistick Murulectere sec00 5xs:99 s35ida sputed
-
Assume todays settlement price on a CME EUR futures contract is $1.3180 per euro. You have a long position in one contract. EUR125,000 is the contract size of one EUR contract. Your performance bond...
-
Q2. Company ABC bought an equipment for $20,000 in 2015, with useful life of 5 years $5,000 residual value amortized using straight-line method. Prepare a table to illustrate the differences...

Study smarter with the SolutionInn App


