A mixture of sulfur dioxide and oxygen gas reacts as shown below. (a) Write the balanced
Question:
A mixture of sulfur dioxide and oxygen gas reacts as shown below.
(a) Write the balanced equation (remember to express the coefficients as the lowest set of whole numbers).
(b) Name the product.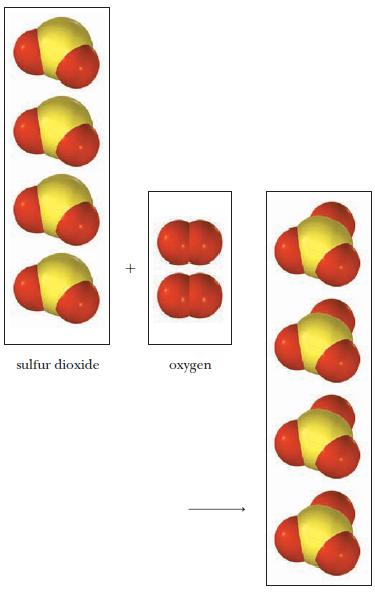
Transcribed Image Text:
sulfur dioxide oxygen
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Completed and balanced c...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A mixture of carbon monoxide and oxygen gas reacts as shown below. (a) Write the balanced equation (remember to express the coefficients as the lowest set of whole numbers). (b) Name the product....
-
Iron pyrite, FeS 2 , is the form in which much of the sulfur exists in coal. In the combustion of coal, oxygen reacts with iron pyrite to produce iron(III) oxide and sulfur dioxide, which is a major...
-
Sulfuric acid is manufactured by the Contact process, in which a mixture of sulfur dioxide and excess air, preheated to 450C, is passed over a catalyst. a. How is the sulfur dioxide made? Give an...
-
discuss case study a bout remote analysis during covid 1 9 - 1 9 virus
-
Find the input impedance of a short-circuited coaxial transmission line of figure if Z o = 65 + j38?, ? = 0.7 + j2.5 /m, ? = 0.8 m. 0
-
Holly Springs, Inc. contracted with Coldwater Corporation to have constructed a custom-made lathe. The machine was completed and ready for use on January 1, 2018. Holly Springs paid for the lathe by...
-
The variance on account of reasons other than mix is termed as Mix Variance.
-
On September 30, 2017, Ericson Company negotiated a two-year, 1,000,000 dudek loan from a foreign bank at an interest rate of 2 percent per year. It makes interest payments annually on September 30...
-
Static budget versus flexible budget The production supervisor of the Machining Department for Hagerstown Company agreed to the following monthly static budget for the upcoming year: Hagerstown...
-
The percentage changes in prepaid expenses and other current assets jumped up 16.5% in scal 2014 and then fell by 35.2% in scal 2015. Did the changes in the dollar amounts of this account have a huge...
-
Write balanced equations for the following reactions. (a) C5H12 + O CO + HO (b) NH3 + O N + HO (c) KOH + HSO4 KSO4 + HO
-
Given the following equation, calculate the mass (in grams) of AlCl 3 that can be produced from 4.40 g Al and 12.0 g Cl 2 ? 2A1+3C1 2A1C13
-
Describe what purchasing managers do. LO.1
-
How much Group Revenue do you currently have booked for September 2024? (format $, no decimals; e.g, $5,000) How much additional Group Revenue do you need to book to hit your budget for September...
-
What strategies do businesses have in place that promote equal opportunity within the organization? Do most businesses offer career development and training to its employees? If so, why? How do...
-
How do you do the step method in cost accounting? I need help understanding the difference between the step method and reciprocal method.
-
1- How do long-term care changing the health care system in the 21st century? What ethical issues do you see playing a role in this situation? 2- Why do long-term health care and palliative care...
-
How do the processes and layouts of Harley Davidson enable it to deliver goods and services to its customer? (How do they do what they do with the resources they have?)
-
Lysozyme is an antibacterial enzyme that hydrolyzes polysaccharides in bacterial cell walls. It also catalyzes the hydrolysis of a -1,4-linked hexasaccharide oligomer of N-acefylglucosamine into a...
-
Why do bars offer free peanuts?
-
Predict the major product obtained when each of the following compounds is treated with fuming sulfuric acid: (a) Chlorobenzene (b) Phenol (c) Benzaldehyde (d) Ortho-Nitrophenol (e) Para-Bromotoluene...
-
For each of the following groups, identify whether it is an activator or a deactivator, and determine its directing effects: (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) -OMe
-
Starting with benzene and using any other necessary reagents of your choice, design a synthesis for each of the following compounds: (a) (b) (c) Br-
-
A contractor constructed a house for resale, which was sold immediately. For tax purposes, this is an example of A) capital income. B) business income. C) other income. D) property income.
-
You invest $100 in a risky asset with an expected rate of return of 0.12 and a standard deviation of 0.15 and a T-bill with a rate of return of 0.05. What percentages of your money must be invested...
-
Nanometrics, Inc., has a beta of 3.43. If the market return is expected to be 13.50 percent and the risk-free rate is 7.00 percent, what is Nanometrics required return? (Round your answer to 2...

Study smarter with the SolutionInn App


