Identify the carboxylic acid and the alcohol that are necessary in order to make each of the
Question:
a.
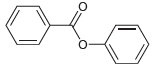
b.
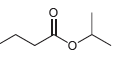
c. CH3CH2CO2C (CH3)3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (19 reviews)
a b c C...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the carboxylic acid chloride that might be used in a Friedel-Crafts acylation reaction to prepare each of the followingacylbenzenes: (b) (a)
-
Oxidation of a primary alcohol to an aldehyde usually gives some over-oxidation to the carboxylic acid. Assume you have used PCC to oxidize pentan-1-ol to pentanal. (a) Show how you would use...
-
An ester is a compound formed by a condensation reaction between a carboxylic acid and an alcohol. Read the discussion of esters in Section 24.4 and then give an example of a reaction forming an...
-
Use Eq. (6) to estimate Af = f(3.53, 8.98) - f(3.5,9)
-
What are Equal Exchange's competitively important resources and capabilities? Which of its resources have the greatest competitive power? Are any of its resources and capabilities able to pass all...
-
t League Apparel Ltd is a large garment manufacturer that operates through two manufacturing stores in Australia (Queensland and New South Wales (NSW)). The following data relates to the Queensland's...
-
Cost and financial accounts are reconciled under non-integral accounting.
-
At the end of the current year, the accounts receivable account of Parkers Nursery Supplies has a debit balance of $350,000. Credit sales are $2,300,000. Record the end-of-period adjusting entry on...
-
Instructions Dec 31, 2016 Dec 31, 2015 2 Assets Accounts receivable et 5643.400.00 566.800.00 1091.000.00 Inventor 5679.400.00 542,400.00 32.800.00 240,000.00 Invest 000 Land 0.00 520.000.00...
-
Jasmine Traders is a home and nursery centre located in Johannesburg. Jasmine Traders is having trouble working out how much they owe Plant World, one of their suppliers as at the end of April. You...
-
How does the CAD designer use a drawing to actually create a finished product?
-
Determine the structures of compounds A through F: Na,Cr,0, H2SO4, H20 [H'] A EtO soch 1) LIAI(OR)H `2) H20 xS NH3
-
A uniform disk with a mass of 4 kg and a radius of 0.2 m is rotating with a rotational velocity of 20 rad/s. a. What is the rotational inertia of the disk? (See fig. 8.15.) b. What is the angular...
-
1. What are the threats being faced by Indian General Insurance Ltd. (IGIL)? 2. What are its traditional strengths? What 'business definitions' should it follow while capitalizing on its traditional...
-
You go to discuss the incident and the client's claims with your supervisor. As you retell the incident, it is clear that your supervisor is not comfortable. You ask your supervisor for advice on the...
-
Case Study Two: Rawlings Rawlings is an American sports equipment manufacturing company based in Town and Country, Missouri, and founded in 1887. Rawings specializes in baseball equipment and...
-
The discussion is for Administrating organizational change course. (we should write 300 words) Discussion question is: Refer to table 6.4 in your book. Think of a time when you were introduced to...
-
Content: Identify at least two resources for each of the four critical sections in the course project: Strategic Planning, Healthcare Reimbursement, Revenue Cycle Process, and Reimbursement...
-
Solve the equation x 3 - 9x 2 + 26x - 24 = 0 given that 4 is a zero of f(x) = x 3 - 9x 2 + 26x - 24.
-
What is taxable income, and what is the formula for determining taxable income?
-
A compound A (C6H) undergoes catalytic hydrogenation over Lindlar catalyst to give a compound B, which in turn undergoes ozonolysis followed by workup with aqueous H2O2 to yield succinic acid and two...
-
A compound A (C6H) undergoes catalytic hydrogenation over Lindlar catalyst to give a compound B, which in turn undergoes ozonolysis followed by workup with aqueous H2O2 to yield succinic acid and two...
-
Complete the reactions given in Fig. P14.45 using knowledge or intuition developed from this or previous chapters. (a) (b) CH CH MgBr DO CH,CH CH CH2 CH 0 CH CH2-O S-O- CH2CH3 diethvl sulfate
-
Provide a graph chart or data with sample numbers indicating Valuing Stocks and Bonds?
-
I just need help with part b. It says that the answer is not complete and some are wrong. So can you kindly fix it for me and give me the full answers as it says the answer is "not complete". Thank...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...

Study smarter with the SolutionInn App


