A mixture of hydrogen and nitrogen gas reacts as shown in the drawing below. (a) Write
Question:
A mixture of hydrogen and nitrogen gas reacts as shown in the drawing below.
(a) Write the balanced equation.
(b) Which reactant is the limiting reactant?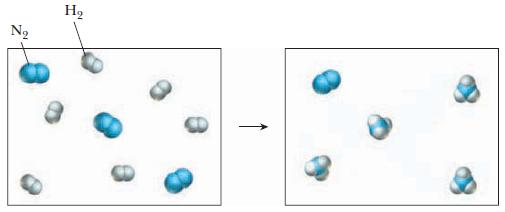
Transcribed Image Text:
N₂ H₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a 3H...View the full answer

Answered By

AKANKSHA CHATURVEDI
I am a organized professional with proven teaching guidance,and counseling skills.possess a strong track record in improving test scores and teaching effectively.I have ability to be a team player and resolve problems and conflicts professionally.Have an ability to communicate complex information in a simple and intertaining manner. Looking to contribute my knowledge and skills on platform like(solutionInn) that offers a genuine opportunity for career progression.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A mixture of antimony atoms and Cl 2 in the gas phase reacts as shown in the drawing below. (a) Write the balanced equation. (b) Which reactant is the limiting reactant?
-
A mixture of hydrogen and uranium hexafluoride (UF6, molar mass: 352 kg/kmol) is stored in an abandoned oil well (in spherical chambers radioactive uranium can become critical leading to nuclear...
-
Lead(II) sulfide reacts with hydrogen peroxide to give lead(II) sulfate as shown in the unbalanced chemical equation PbS + H 2 O 2 PbSO 4 + H 2 O If 63.2 g of PbS is reacted with 48.0 g of H 2 O 2 ,...
-
The number of hours of daylight that occur at any location on Earth depends on the time of year and the latitude of the location. The equations below model the numbers of hours of daylight in Seward,...
-
A cotton reel is made up of a hub of radius a and two end caps of radius b. The mass of the complete reel is m and its moment of inertia about its longitudinal axis is I. The reel rests on a...
-
Direct Materials Variances Bellingham Company produces a product that requires 15 standard pounds per unit. The standard price is $8 per pound. If 2,800 units used 43,300 pounds, which were purchased...
-
Red meat and heart disease. Refer to the study presented at ESC Preventive Cardiology 2021 that explored the link between red meat and heart disease, Exercise 9.12 (p. 533). Recall that the study...
-
1. Would you describe the exposure of the Sports Exports Company to exchange rate risk as transaction exposure? Economic exposure? Translation exposure? 2. Jim Logan is considering a change in the...
-
In 2020, Flowers Corp., a U.S.-based company, reported $245,000 in before-tax foreign income. Their foreign income stays in a subsidiary in Canada, where tax rate was 15%. U.S. tax rate was 21%. a)...
-
Name: Marks Sections Journal Type # of Questions 57 G/L Accounts 68 Trial Balance Income Statement Statement of Owners Equity 20 13 6 Balance Sheet 12 TOTAL 176 Once complete, submit as instructed....
-
In a reaction of HCl and NaOH, the theoretical yield of H 2 O is 78.2 g. What is the theoretical yield of NaCl?
-
Lithium metal reacts with O 2 to form lithium oxide. What is the theoretical yield of lithium oxide when 0.45 g lithium reacts with excess O 2 ?
-
If the government provides healthcare to achieve the efficient coverage, how many families are covered and how much must taxpayers pay? The marginal cost of insurance is a constant $6,000 per family...
-
Concord Timber Company owns 9,000 acres of timberland purchased in 2014 at a cost of $1.470 per acre. At the time of purchase. the land without the timber was valued at $420 per acre. In 2015,...
-
Foofy computes z-scores for a set of normally distributed exam scores. She obtains a z-score of -3.96 for 8 out of 20 of the students. What do you conclude?
-
Part 1 Recording Using the financial statements for the hypothetical company - Big Box Retailer-record the transactions for the year to the financial statement. The financial statements may be found...
-
Finding Standard Deviation from a Frequency Distribution. In Exercises 37-40, refer to the frequency distribution in the given exercise and compute the standard deviation by using the formula below,...
-
STAR Co. provides paper to smaller companies whose volumes are not large enough to warrant dealing directly with the paper mill. STAR receives 100-feet-wide paper rolls from the mill and cuts the...
-
The following data are taken from an unadjusted trial balance at December 31, 2016: Prepaid rent $600 Office supplies 700 Income taxes payable -0- Unearned commissions revenue 1500 Salaries expense...
-
Which of the companies has the lowest accounts receivable turnover in the year 20X2? a. Company A. b. Company B. c. Company C. d. CompanyD. 20X1 20X2 Credit Sales Average Receivables Balance $1.0...
-
In Problem 7.4, we saw that an intramolecular substitution reaction can occur when the nucleophilic center and electrophilic center are present in the same compound. Draw the transition state of the...
-
Treatment of 5-hexen-1-ol with bromine affords a cyclic product: The mechanism of this reaction involves several steps, one of which is an intramolecular S N 2 process: In this step, a bond is in the...
-
Nicotine is an addictive compound found in tobacco, and choline is a compound involved in neurotransmission. The biosynthesis of each of these compounds involves the transfer of a methyl group from...
-
DOLLAR TREE GROCERY OUTLET Short-Term Liquidity 2021 2022 2021 2022 Current Ratio 1.35 1.51 1.86 1.67 Quick Ratio 0.24 0.15 0.63 0.42 Cash Ratio Cash Conversion Cycle 34.78 45.75 19.41 21.61 Days...
-
A family has a $117,443, 25-year mortgage at 5.4% compounded monthly. (A) Find the monthly payment and the total interest paid. (B) Suppose the family decides to add an extra $100 to its mortgage...
-
Comparing the actual and planned cost of a consulting engagement completed by an engineering firm such as Allied Engineering.

Study smarter with the SolutionInn App


