Aspirin, or acetylsalicylic acid, has the formula C 9 H 8 O 4 and the skeleton structure
Question:
Aspirin, or acetylsalicylic acid, has the formula C9H8O4 and the skeleton structure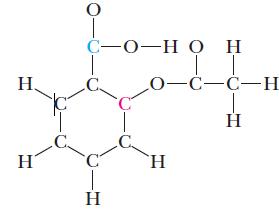

Transcribed Image Text:
. H C-O-H 0 TT 0-C-C-H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To answer these questions lets take them one at a time a Completing the Lewis structure for aspirin and identifying the number of sigma bonds and pi b...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
An aspirin tablet contains 325 mg of acetylsalicylic acid (C 9 H 8 O 4 ). How many acetylsalicylic acid molecules does it contain? SORT You are given the mass of acetylsali- cylic acid and asked to...
-
The active ingredient in aspirin is acetylsalicylic acid. A 2.51-g sample of acetylsalicylic acid required 27.36 mL of 0.5106 M NaOH for complete reaction. Addition of 15.44 mL of 0.4524 M HCl to the...
-
The active ingredient in aspirin is acetylsalicylic acid (HC9H7O4), a monoprotic acid with Ka = 3.3 ( 10-4 at 25 (C. What is the pH of a solution obtained by dissolving two extra-strength aspirin...
-
Draw and name the eight isomeric alcohols with formula C5H12O.
-
Current Ratio and Quick Ratio In recent years, Dixie Co. has greatly increased its current ratio. At the same time, the quick ratio has fallen. What has happened? Has the liquidity of the company...
-
During the year, Thompson Plastics was in negotiations with the local union over wages. A settlement was finally reached, and the average wage per hour was increased to $32. Production fell to...
-
Consider the population described by the probability distribution shown below. The random variable x is observed twice. If these observations are independent, verify that the different samples of...
-
Maloney Pharmaceuticals manufactures an over-the-counter allergy medication called Breathe and is trying to win market share from Sudafed and Tylenol. Maloney Pharmaceuticals has developed several...
-
5-year CDX NA IG index is quoted by a market maker as bid 160 bp, ask (offer) 165 bp. This index includes 125 North American investment-grade companies. Suppose you want $800,000 of protection for...
-
The ions ClF - 2 and ClF + 2 have both been observed. Use the VSEPR model to predict the FClF bond angle in each.
-
Recently, the structure of an amine compound, NR 3 (R = large organic group), has been determined to have CNC bond angles of 119.2 degrees. It is believed that the bond angles of about 109 degrees...
-
Have you had an interview in the past, and the interviewer never got back to you? Or have you sent a rsum and not received a response? Think about one of these situations, and write an email to...
-
Given forecast errors of 4, 8, and -3, what is the MAD? What is the MSE?
-
Padgett Rentals can purchase a van that costs \($48,000\) ; it has an expected useful life of three years and no salvage value. Padgett uses straight-line depreciation. Expected revenue is...
-
Rainwater Corp. expects to sell 600 umbrellas in May and 400 in June. Each umbrella sells for \($15\). Rainwaters beginning and ending finished goods inventories for May are 75 and 50 units,...
-
Don Moon is the owner of ABC Cleaning. At the beginning of the year, Moon had \(\$ 2,400\) in inventory. During the year, Moon purchased inventory that cost \(\$ 13,000\). At the end of the year,...
-
Agua Ole is a distributor of bottled water. For each of items a through c, compute the amount of cash receipts or payments Agua Ol will budget for September. The solution to one item may depend on...
-
A separation of 2.5 mg of an unknown mixture has been optimized on a column of length L and diameter d. Explain why you might not achieve the same resolution for 5.0 mg on a column of length 2L and...
-
Assume that a trial balance is prepared with an account balance of $21,360 listed as $21,630 and an account balance of $1,500 listed as $15,000. Identify the transposition and the slide.
-
Determine the absolute maximum bending stress in the 2-in.-diameter shaft. There is a journal bearing at A and a thrust bearing at B. 900 Ib 300 lb 12 in. 24 in. 18 in.
-
Determine the absolute maximum bending stress in the beam, assuming that the support at B exerts a uniformly distributed reaction on the beam. The cross section is rectangular with a base of 3 in....
-
Determine, to the nearest millimeter, the smallest allowable diameter of the shaft which is subjected to the concentrated forces. There is a journal bearing at A and a thrust bearing at B. The...
-
Ray Company provided the following excerpts from its Production Department's flexible budget performance report. Required: Complete the Production Department's Flexible Budget Performance Report....
-
Problem 1 5 - 5 ( Algo ) Lessee; operating lease; advance payment; leasehold improvement [ L 0 1 5 - 4 ] On January 1 , 2 0 2 4 , Winn Heat Transfer leased office space under a three - year operating...
-
Zafra and Stephanie formed an equal profit- sharing O&S Partnership during the current year, with Zafra contributing $100,000 in cash and Stephanie contributing land (basis of $60,000, fair market...

Study smarter with the SolutionInn App


