Balance these reactions. (a) Al(s) + O(g) AlO3(s) (b) N(g) + H(g) NH3(g) (c) CH%(l)
Question:
Balance these reactions.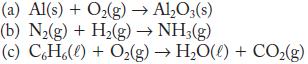
Transcribed Image Text:
(a) Al(s) + O₂(g) → Al₂O3(s) (b) N₂(g) + H₂(g) → NH3(g) (c) CH%(l) + Oz(g) → H,O(l) + CO,(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Lets balance the given chemical equations a Als O2g Al2O3s This eq...View the full answer

Answered By

Emily Grace
With over a decade of experience providing top-notch study assistance to students globally, I am dedicated to ensuring their academic success. My passion is to deliver original, high-quality assignments with fast turnaround times, always striving to exceed their expectations.
4.90+
3+ Reviews
24+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Balance these redox reactions by the half reaction method. a. Ca + H+ ( Ca2+ + H2 b. Sn2+ ( Sn + Sn4+ (Hint: both half reactions will start with the same reactant.)
-
Balance these redox reactions by the half reaction method. a. Fe3+ + Sn2+ ( Fe + Sn4+ b. Pb2+ ( Pb + Pb4+ (Hint: both half reactions will start with the same reactant.)
-
Balance these redox reactions by inspection. a. Na + F2 ( NaF b. Al2O3 + H2 ( Al + H2O
-
Describe the Economic Analysis of the Valero Energy Corporation. Demonstrate Valero Energy Corporation is susceptible to Macroeconomic outlook both in the U.S.& foreign markets
-
Three lossless lines are connected as shown in Figure. Determine Zin. 3A/4- SA/8 SA/8 /2- Z, = 75 2 z, - 100 2 Zia Z, - 60 - j 35 Z,= 502
-
Suppose Seafood House restaurant is considering whether to (1) bake bread for its restaurant in-house or (2) buy the bread from a local bakery. The chef estimates that variable costs of making each...
-
A cost variance is said to be favourable if the standard costing is more than the actual cost.
-
On January 1, 2016, Hart Company issued bonds with a face value of $150,000, a stated rate of interest of 8 percent, and a five-year term to maturity. Interest is payable in cash on December 31 of...
-
(Use Excel) Will Co. is expected to pay a dividend of $4 per share at the end of year -1(D1) and the dividends are expected to grow at a constant rate of 4.5% forever. If the current price of the...
-
How many cycles are required for the pipelined MIPS processor to issue all of the instructions for the program in Exercise 7.23? What is the CPI of the processor on this program?? Data from Problem...
-
(a) Write the equation for perchloric acid (HClO 4 ) dissolving in water. (b) Write the equation for sodium nitrate dissolving in water.
-
Write balanced equations for the following reactions. (a) NH4 + NO4N + HO (b) F + HO HF + O (c) NaO + HO NaOH
-
Let \(p_{1}=\operatorname{Pr}(y=1 \mid x=1)=0.8\) and \(p_{2}=\operatorname{Pr}(y=1 \mid x=0)=0.4\). (a) Compute the relative risk of response \(y=1\) of population \(x=1\) to population \(x=0\), and...
-
Scenario : Wanda, a BCBA, is updating an intervention plan for a leaner on her caseload to submit for insurance funding authorization. Part of the plan includes the completion of an adaptive...
-
a) how can your company accommodate generational or gender difference within your company ? b) how can your company accommodate communication and or language difference within your company ?
-
3. Consider the following data for two catalysts, A and B. The temperature is 25 C and the reaction occurs at standard conditions. a. Make a Tafel plot and determine the Tafel slope. Estimate the...
-
How consumption can be helpful in facilitating the construction of your identity? Explain the ways in which the symbolic meanings, connected with your consumption choices are important to you? Is...
-
Cataumet Boats, Inc. Jaime Giancola had just completed the first half of her MBA program and wanted to work on a project during the summer that would give her some practical experience applying what...
-
Predict the result that would have been expected in the experiment described by Eq. 27.28 for an antarafacial migration.
-
Problem 2. (0.6 points, 0.2 points for each question) (a) A company turns its inventory 2 times a month. Its months-of-supply = Its days-of-supply = Please show your analysis below: _months. days. (1...
-
You wish to design an effusion source for Br atoms from Br 2 (g). If the source is to operate at a total pressure of 7.5 Torr, what temperature is required to produce a degree of dissociation of...
-
Calculate G for the isothermal expansion of 2.25 mol of an ideal gas at 325 K from an initial pressure of 12.0 bar to a final pressure of 2.5 bar.
-
When ortho-bromonitrobenzene is treated with NaOH at elevated temperature, only one product is formed. (a) Draw the product. (b) Identify the intermediate formed en route to the product. (c) Would...
-
American Food Services, Incorporated leased a packaging machine from Barton and Barton Corporation. Barton and Barton completed construction of the machine on January 1 , 2 0 2 4 . The lease...
-
Which of the following statements is true? Financial measures tend to be lag indicators that report on the results of past actions. LA profit center is responsible for generating revenue, but it is...
-
Andretti Company has a single product called a Dak. The company normally produces and sells 8 0 , 0 0 0 Daks each year at a selling price of $ 5 6 per unit. The company s unit costs at this level of...

Study smarter with the SolutionInn App


