Write balanced equations for the following reactions. (a) NH4 + NO4N + HO (b) F + HO
Question:
Write balanced equations for the following reactions.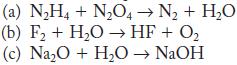
Transcribed Image Text:
(a) N₂H4 + N₂O4N₂ + H₂O (b) F₂ + H₂O → HF + O₂ (c) Na₂O + H₂O → NaOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 2N 2 H 4 N 2 O ...View the full answer

Answered By

Brown Arianne
Detail-oriented professional tutor with a solid 10 years of experience instilling confidence in high school and college students. Dedicated to empowering all students with constructive feedback and practical test-taking strategies. Effective educator and team player whether working in a school, university, or private provider setting. Active listener committed to helping students overcome academic challenges to reach personal goals.
4.60+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write balanced nuclear equations for the following reactions and identify X:
-
Write balanced nuclear equations for the following reactions and identify X: 80 34
-
Write balanced equations for each of the following reactions (some of these are analogous to reactions shown in the chapter). (a) Aluminum metal reacts with acids to form hydrogen gas. (b) Steam...
-
Data for Barry Computer Co. and its industry averages follow. a. Calculate the indicated ratios for Barry. b. Construct the DuPont equation for both Barry and the industry. c. Outline Barrys...
-
A lossless transmission line with a characteristic impedance of 75 is terminated by a load of 120. The length of the line is 1.25. If the line is energized by a source of 100 V (rms) with an internal...
-
Some recent financial statements for Smolira Golf Corporation follow. 2020 2021 Assets Current assets Cash Accounts receivable Inventory SMOLIRA GOLF CORPORATION 2020 and 2021 Balance Sheets 2020...
-
Material yield variance is favourable if the standard output is more than the actual output.
-
High Company issued $100,000 face value of bonds on January 1, 2012. The bonds had a 5 percent stated rate of interest and a 10-year term. Interest is paid in cash annually, beginning December 31,...
-
QUESTION 1 [ 2 0 MARKS ] Sam Sithole is a resident of the Republic. He is 4 7 years old and is married in community of property. The following information relates to the 2 0 2 3 year of assessment...
-
Gapco manufactures three products, whose daily labor and raw material requirements are given in the following table. The profits per unit of the three products are $25, $30, and $22, respectively....
-
Balance these reactions. (a) Al(s) + O(g) AlO3(s) (b) N(g) + H(g) NH3(g) (c) CH%(l) + Oz(g) H,O(l) + CO,(g)
-
In an experiment performed in the laboratory, 44 g NH 3 is mixed with 120 g O 2 , and 73 g NO is isolated. Given the following equation, what is the percent yield? 4NH3 + 50 4NO + 6HO
-
A fund receives investments at the beginning of each year and generates returns for three years as follows: Which return measure over the three-year period is negative? A. Geometric mean return B....
-
Your task is to check the internet and the?Common Vulnerabilities and Exposures (CVE) List?for networked IoT or?IoMT?devices with publicly known problems identified in the past six months.?? Select...
-
The first quarter tax return needs to be filed for Prevosti Farms and Sugarhouse by April 15, 2021. For the taxes, assume the second February payroll amounts were duplicated for the March 5 and March...
-
ABC Boating had a large fire that destroyed many of their boats ready to go to market. ABC decided to lay off a large number of employees rather than continue to pay them. The wages that this group...
-
HOW BEER IS MADE The beer-making process is an art. It takes time, patience and quite a bit of experimentation to find that perfect flavour. Once you have found the right mix, you can replicate it,...
-
Justine, an underwriter at NewWorld Insurance is working with her company's premium auditing department. Which one of the following is true regarding this collaboration? Available answer options...
-
Classify the following sigmaffopic reactions with bracketed number.
-
What are the key dimensions of critical thinking 2. Watch the NBC Learn video on Diet Scams. What types of claims are made in this video Are they valid Elaborate on your responses. Discuss this video...
-
Predict the change in the partial pressure of CO2 as Xe gas is introduced into the reaction vessel at constant volume and temperature. CO(g) + 1/2O 2 (g) CO 2 (g) at equilibrium for which H o R =...
-
You place 3.00 mol of NOCl(g) in a reaction vessel. Equilibrium is established with respect to the decomposition reaction NOCl(g) NO(g) + 1/2Cl 2 (g). a. Derive an expression for K P in terms of the...
-
Predict the change in the partial pressure of CO2 as a platinum catalyst is introduced into the reaction vessel at constant volume and temperature. CO(g) + 1/2O 2 (g) CO 2 (g) at equilibrium for...
-
An estimated 84 percent of enterprises now use cloud computing solutions involving multiple clouds, whereas less than 10 percent of large organizations employ just a single public cloud. Group of...
-
XYZ inc. was involved in a tax dispute with the national tax authority. The companys legal counsel estimates that there is a 75% likelihood that the company will lose the dispute and that the amount...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?

Study smarter with the SolutionInn App


