Calculate H , S , and G for each of the following reactions at 298 K.
Question:
Calculate ΔH °, ΔS °, and ΔG ° for each of the following reactions at 298 K. State whether the direction of spontaneous reaction is consistent with the sign of the enthalpy change, the entropy change, or both. Use Appendix G for data.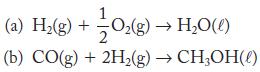
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a AH28583 kJ AS16318 JK AG2372 kJ direction ...View the full answer

Answered By

Ann Wangechi
hey, there, paying attention to detail is one of my strong points, i do my very best combined with passion. i enjoy researching since the net is one of my favorite places to be and to learn. i am a proficient and versatile blog, article academic and research writing i possess excellent English writing skills, great proof-reading. i am a good communicator and always provide feedback in real time. i'm experienced in the writing field, competent in computing, essays, accounting and research work and also as a Database and Systems Administrator
4.90+
151+ Reviews
291+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Calculate H , S , and G for each of the following reactions at 298 K. State whether the direction of spontaneous reaction is consistent with the sign of the enthalpy change, the entropy change, or...
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard reaction entropy for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction...
-
Calculate the standard enthalpy, entropy, and Gibbs free energy for each of the following reactions at 298 K by using data in Appendix 2A. For each case, confirm that the value obtained from the...
-
CPA firm brings in a yoga instructor during the tax busy season to help relieve stress of the employees. Which is true about the CPA firm's ability to take a deduction for the yoga instructor's...
-
Heating carvone with aqueous sulfuric acid converts it into Carvacrol. Propose a mechanism for theisomerization. H2SO4 Heat " Carvacrol Carvone
-
The Willow River Mining Company mines and ships coal. It has experienced the following demand for coal during the past eight years: Year Coat Sales (tons) 1 ...........4260 2 ...........4510 3...
-
3. On the books of Pam Corporation, the investment account is properly reflected on December 31, 2016, at: a $3,240 b $3,264 c $3,276 d Not enough information is given.
-
A 0.25-m3 insulated pistoncylinder device initially contains 0.7 kg of air at 20°C. At this state, the piston is free to move. Now air at 500 kPa and 70°C is allowed to enter the cylinder...
-
Statement of stockholders equity Noric Cruises Inc. began the month of October with the following balances: Common Stock, $ 1 4 0 , 0 0 0 ; Additional Paid - In Capital, $ 3 , 5 0 0 , 0 0 0 ; and...
-
Calculate H , S , and G for each of the following reactions. State whether the direction of spontaneous reaction is consistent with the sign of the enthalpy change, the entropy change, or both. Use...
-
Calculate G for the following reactions two diff erent ways: (1) Use Hesss law and the standard Gibbs free energies of formation, (2) Use G = H - TS. Compare the two values and judge whether you get...
-
SEK Corp. factors $400,000 of accounts receivable with Mays Finance Corporation on a without recourse basis on July 1, 2012. The receivables records are transferred to Mays Finance, which will...
-
2. (3 points) NextGames Inc. has a new video game cassette for the upcoming holiday season. It is 3 trying to determine the target cost for the game if the selling price per unit will be set at $130,...
-
1. After watching the SR WEBINAR on how risk managers create better decision-making through a positive culture what do you think the three (or more) important points made during the webinar 2....
-
| Variance analysis, multiple products. The Robin's Basket operates a chain of Italian gelato stores. Although the Robin's Basket charges customers the same price for all flavors, production costs...
-
Question 31 of Your local coffee shop is extremely busy, so the cashier asks what you'd like to order and your name. The cashier writes this information onto a cup and passes it to the barista. After...
-
Find SSR = xy Rx+1 -dA, R= [0,2] x [4,4] Round your answer to four decimal places.
-
One way to measure ionization energies is ultraviolet photoelectron spectroscopy (UPS, or just PES), a technique based on the photoelectric effect. In PES, monochromatic light is directed onto a...
-
Evaluate the line integral, where C is the given curve. C x 2 dx + y 2 dy, C consists of the arc of the circle x 2 + y 2 = 4 from (2, 0) to (0, 2) followed by the line segment from (0, 2) to (4, 3)
-
A 1.8-kW electric heater takes 15 min to boil a quantity of water. If this is done once a day and power costs 10 cents/kWh, what is the cost of its operation for 30 days?
-
A lightning bolt strikes an airplane with 40 kA for 1.7 ms. How many coulombs of charge are deposited on the plane?
-
Find I and the power absorbed by each element in the network of Fig. 1.30. 44 A 10 A ) 15 V 15 V +. (1+
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...
-
C: The sor at the poopecin 0ieund to twe oxind places)
-
What information may an Appeals Officer not consider when reviewing a taxpayer's case? Select one: a. The cost involved for the IRS to hire an expert witness for litigation. b. Litigation hazards...

Study smarter with the SolutionInn App


