Calculate the enthalpy change observed in the combustion reaction of 1.00 g ethane, using the thermochemical equation.
Question:
Calculate the enthalpy change observed in the combustion reaction of 1.00 g ethane, using the thermochemical equation.
Thermochemical Equation![]()
Strategy
We will use the same approach as in previous stoichiometry calculations. Convert the mass of ethane to moles; then use stoichiometric relations in the thermochemical equation to calculate ΔH.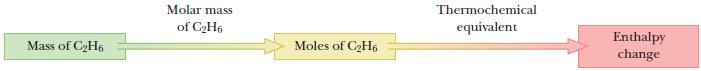
Transcribed Image Text:
2C₂H6(g) + 702₂(g) → 4CO₂(g) + 6H₂O(l) ΔΗ = -3120 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The equation given earlier in this section is First we convert the mass o...View the full answer

Answered By

Mwangi Clement
I am a tried and tested custom essay writer with over five years of excellent essay writing. In my years as a custom essay writer, I have completed more than 2,000 custom essays in a diverse set of subjects. When you order essays from me, you are working with one of the best paper writers on the web. One of the most common questions I get from customers is: “can you write my essay?” Upon hearing that request, my goal is to provide the best essays and overall essay help available on the web. I have worked on papers in subjects such as Nursing and Healthcare, English Literature, Sociology, Philosophy, Psychology, Education, Religious Studies, Business, Biological Sciences, Communications and Media, Physical Sciences, Marketing and many others. In these fields, my specialties lie in crafting professional standard custom writings. These include, but are not limited to: research papers, coursework, assignments, term papers, capstone papers, reviews, summaries, critiques, proofreading and editing, and any other college essays.
My extensive custom writings experience has equipped me with a set of skills, research abilities and a broad knowledge base that allows me to navigate diverse paper requirements while keeping my promise of quality. Furthermore, I have also garnered excellent mastery of paper formatting, grammar, and other relevant elements. When a customer asks me to write their essay, I will do my best to provide the best essay writing service possible. I have satisfactorily offered my essay writing services for High School, Diploma, Bachelors, Masters and Ph.D. clients.
I believe quality, affordability, flexibility, and punctuality are the principal reasons as to why I have risen among the best writers on this platform. I deliver 100% original papers that pass all plagiarism check tests (Turnitin, Copyscape, etc.). My rates for all papers are relatively affordable to ensure my clients get quality essay writing services at reasonable prices.
4.50+
5+ Reviews
14+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Circle the functional groups that are eliminated in the formation of a peptide bond between the amino acids shown below. Draw the structure of the dipeptide. R1 H R2 H C-C-N H C-C-N H
-
The chapter introduction introduced the following reaction as one chemical reaction used to launch the space shuttle. Calculate the mass of aluminum required to generate 60,500 kJ energy (enough to...
-
Th e chemical industry converts hydrocarbons of low molecular mass to larger and more useful compounds. Calculate the change in enthalpy for the synthesis of cyclohexane (C 6 H 12 ), a compound used...
-
Joseph Thompson is president and sole shareholder of Jay Corporation. In December 2019, Joe asks your advice regarding a charitable contribution he plans to have the corporation make to the...
-
A reverse-osmosis plant is used to treat 30,000,000 gal/day of seawater at 20C containing 3.5 wt% dissolved solids to produce 10,000,000 gal/day of potable water with 500 ppm of dissolved solids, and...
-
Use the data below to do the following: -Indicate next to each item if it is an in flow an outflow or other -Indicate next to each item if it is an operating activity, financing activity, asset...
-
What is a scrap report and when is it used? LO.1
-
The graph of v = f(x) is given. Match each equation with its graph and give reasons for your choices. (a) y = f (x - 4) (b) y = f (x) + 3 (c) y = 1/3 f(x) (d) y = - f (x + 4) (e) y = 2f(x + 6) en
-
Using the following categories, indicate the effects of the following transactions. Indicate the accounts affected and the amounts. A. During the period, customer balances are written off in the...
-
Consider the idealized, symmetric two-cell wing box shown in the figure below. The area enclosed by the semi-elliptical web 45 is 51,500 mm. The shear modulus of the material is G = 27 GPa....
-
Why must the physical states of all reactants and products be specified in a thermochemical equation?
-
Use Equation 5.2 to calculate the enthalpy change when 5.00 g O 2 is consumed by reaction with N 2 , forming NO. Equation 5.2 N(g) + O(g) - 2NO(g) = +181.8 kJ
-
Refer to problem 6-23, at the end of Chapter 6, concerning the confirmation of accounts receivable of Pittance Manufacturing Company. You are now considering using dollar unit sampling in the audit...
-
1. Identify an industry that competes internationally (i.e., fast food, clothing, sportswear, automotive, etc). All your companies must be from ONE Industry (you cannot discuss Taco Bell and Nike)....
-
A research article on " Leadership in Project Management: Cultivating Strong Employee-Employer Bonds" shows major findings on why big companies fail in leadership skill practice. How they can...
-
Discuss and Identify the current types of stock, such as common or preferred stock, currently issued, and outstanding. Include a narrative description along with the values and number of shares found...
-
The organization we intend to study is Local Point, a student cafeteria run by UW Housing & Food Services. Our team would like to figure out how to utilize modern technology and rational...
-
Briefly summarize the Coase Theorem (include the 3 key conditions). List the major types of approaches government typically takes to deal with negative externalities. Suppose the demand for...
-
Cardinality ratios often dictate the detailed design of a database. The cardinality ratio depends on the real-world meaning of the entity types involved and is defined by the specific application....
-
As you rewrite these sentences, replace the cliches and buzzwords with plain language (if you don't recognize any of these terms, you can find definitions online): a. Being a jack-of-all-trades, Dave...
-
Rank this group of compounds in order of increasing boiling point. -NH2 -N-
-
Identify whether each of the following compounds is expected to be water soluble: (a) (b) (c) -NH2 -NH2
-
For each of the following pairs of compounds, identify which compound is the stronger base: (a) (b) (c) (d) N- N- N- N-
-
Ellis Perry is an electronics components manufacturer. Information about the company's two products follows: \ table [ [ , , , ] , [ Units produced,AM - 2 , FM - 9 , ] , [ Direct labor hours required...
-
Which of the following requirements to claim Earned Income Tax Credit is TRUE? The credit can be claimed under any filing status. The taxpayer must have a valid SSN for employment in the U.S., issued...
-
Olde Tyme Beverage Companys operating activities for the year are listed below. Cost of Goods Manufactured $131,000 Operating expenses 80,000 Beginning inventory, FG 16,000 Ending inventory, FG...

Study smarter with the SolutionInn App


