Calculate the equilibrium constant from experimental measurements. (a) Nitrogen dioxide dissociates into nitrogen monoxide and oxygen. 2NO(g)
Question:
Calculate the equilibrium constant from experimental measurements.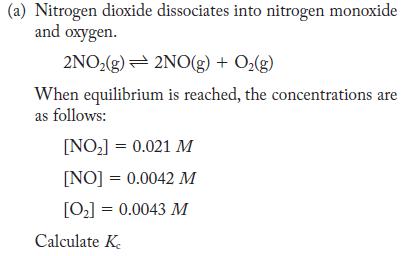
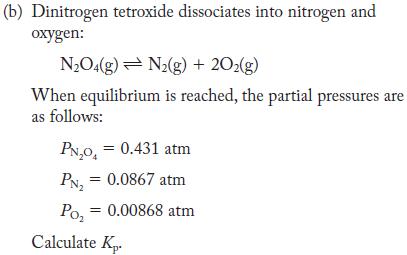
Transcribed Image Text:
(a) Nitrogen dioxide dissociates into nitrogen monoxide and oxygen. 2NO(g) 2NO(g) + O(g) When equilibrium is reached, the concentrations are as follows: [NO] = 0.021 M [NO] = 0.0042 M [0] = 0.0043 M Calculate K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a 17...View the full answer

Answered By

Ehsan Mahmood
I’ve earned Masters Degree in Business Studies and specialized in Accounts & Finance. Couple with this, I have earned BS Sociology from renowned institute of Pakistan. Moreover, I have humongous teaching experience at Graduate and Post-graduate level to Business and humanities students along with more than 7 years of teaching experience to my foreign students Online. I’m also professional writer and write for numerous academic journals pertaining to educational institutes periodically.
4.90+
248+ Reviews
287+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Calculate the equilibrium constant Kc at 25oC from the free-energy change for the following reaction: See Appendix C for data. Zn(s) +2Ag (a)Zn2 (a) Ag(s)
-
Calculate the equilibrium constant Kc for the following reaction from standard electrode potentials. Fe(s) + Sn**(ag) = Fe* (ag) + Sn*(aq)
-
Determine the vector A-C, given the vectors A and C in the figure. (Figure 1) Figure B (B=26.5) 56.0% (A = 44.0) 28.0 C(C= 31.0) 1 of 1 Determine the magnitude of the vector A - . Express your...
-
Capital budgeting has the same focus as accrual accounting. Do you agree? Explain.
-
On September 1 of the current year, Maria Edsall established a business to manage rental property. She completed the following transactions during September: a. Opened a business bank account with a...
-
How do you feel about being given food, t-shirts and praise for working harder? 101-1
-
Refer to the original data in P22-30B and the revisions presented in P22-34B. Requirements 1. Prepare the schedule of budgeted cash collections from customers for April and May. 2. Prepare the...
-
Valuation of common stocks is a fascinating topic. What makes the topic interesting is that net oveyore pronto Using the Gordon Model (constant growth version of the formula for the price of a shared...
-
An equilibrium mixture contains 3.00 mol CO, 2.00 mol Cl 2 , and 9.00 mol COCl 2 in a 50-L reaction flask at 800 K. Calculate the value of the equilibrium constant K c for the reaction at this...
-
Write the expression for the equilibrium constant (K p ) for the following: (a) Cl(g) + HO(g) 2HCl(g) + 0(g) = (b) 2NO(g) NO4(g) (c) 302(g) 203(g) (d) CO(g) CO(g) +0(g) 2
-
What is a hypothesis and do you consider that accounting research should necessarily involve the development of empirically testable hypotheses? LO 1.3 , 1.6
-
How do socio-cognitive mechanisms, such as social identity theory and self-categorization theory, contribute to the formation and maintenance of organizational culture ?
-
How do you Sales Forecast and an Expense forecast for future years?
-
2. Do you really think the Bono case described in Ch. 2 is a genuine ethical conflict? Explain. 6. Describe the ethical issue in the Siemens case
-
How do I calculate using the SPC method if my key metric is time
-
Labor Standards: Where Do They Belong on the International Trade Agenda? Author(s): Drusilla K. Brown Link. https://viu.summon.serialssolutions.com/?#!/search?....
-
Acetic anhydride is formed from acetic acid in a condensation reaction that involves the removal of a molecule of water from between two acetic acid molecules. Write the chemical equation for this...
-
Research an article from an online source, such as The Economist, Wall Street Journal, Journal of Economic Perspectives, American Journal of Agricultural Economics, or another academic journal. The...
-
Consider a skier who coasts up to the top of a hill and then continues down the other side. Draw a qualitative plot of what the skiers speed might look like.
-
The acceleration of an object that falls freely under the action of gravity near the Earths surface is negative and constant. (a) Does the objects instantaneous acceleration equal its average...
-
In the drop zone. Consider a skydiver who jumps from an airplane. Suppose she waits for 1 min before opening her parachute and she lands 4 min after leaving the airplane. Draw qualitative plots of...
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


