Determine the number of sodium ions and chloride ions present in the face-centered cubic unit cell of
Question:
Determine the number of sodium ions and chloride ions present in the face-centered cubic unit cell of sodium chloride.
Strategy
Use Figure 11.27 to count the atoms of each type. Remember that an atom in a corner contributes one eighth of an atom to that unit cell, an atom on a face contributes only half, and an atom on an edge contributes one fourth. Recall also that there is a sodium ion in the center of the unit cell.
Figure 11.27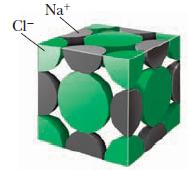
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





