For the same reaction, NO + CO NO + CO is the following two-step mechanism the correct
Question:
For the same reaction,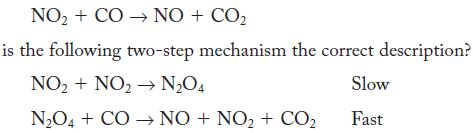
Transcribed Image Text:
NO + CO NO + CO is the following two-step mechanism the correct description? NO + NO NO4 Slow NO4 + CO NO + NO + CO Fast
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The mechanism is consistent with the experimental data because it sums to the ove...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The following graph shows two different reaction pathways for the same overall reaction at the same temperature. (a) Which pathway is slower? Why? (b) How can there be two different reaction pathways...
-
One mechanism for the destruction of ozone in the upper atmosphere is a. Which species is a catalyst? b. Which species is an intermediate? c. E a for the uncatalyzed reaction Is 14.0 kJ. E a for the...
-
One pathway for the destruction of ozone in the upper atmosphere is O3(g) + NO(g) NO2(g) + O2(g) Slow NO2(g) + O(g) NO(g) + O2(g) Fast Overall reaction: O3(g) + O(g) 2O2(g) a. Which species is a...
-
Simplity each of the follewing ratios. f r 15 15kg:350 g 0.45:085 ( 580 ml: L121:104 m/ 40 033:063: 18
-
The propagator in momentum space analogous to (2.5.26) is given by . Derive an explicit expression for for the free- particle case?
-
When Turner Company adopted its defined benefit pension plan on January 1, 2007, it awarded retroactive benefits to its employees. These retroactive benefits resulted in an unrecognized prior service...
-
In what way did using just-in-time inventory management help the supplier, Arvin Sango? LO.1
-
Canton Trade Mart has recently had lackluster sales. The rate of inventory turnover has dropped, and the merchandise is gathering dust. At the same time, competition has forced Cantons suppliers to...
-
Using these data from the comparative balance sheets of Bonita Company, perform a vertical analysis. Using these data from the comparative balance sheets of Bonita Company, perform a vertical...
-
Nitrogen dioxide reacts with carbon monoxide to form carbon dioxide and nitrogen monoxide. The overall stoichiometry is Evaluate the following mechanism to determine whether it is consistent with...
-
Write the expected rate law and molecularity for each of the following elementary reactions in the gas phase. Strategy The rate law for an elementary reaction is derived directly from its...
-
Solve the inequalities. Express the solution sets as intervals or unions of intervals and show them on the real line. Use the result a 2 = |a| as appropriate. x 2 < 2
-
The four forces, 400, 500, 600 and 700N are acting along the edges of a 0.8m cube as shown. Represent the resultant of these forces by 1) A force Fr through the point A 2) A couple moment Mr (give...
-
Problem 1. What is the degree of freedom of the following mechanism? Sliding joint Sliding joint
-
PILAR Manufacturing Co. has three producing departments (P, I, & L), and two service departments (A&R). The total estimated departmental expenses for 2021 before distribution of service department...
-
1. A volleyball player serves the ball at point A with an initial velocity vo at an angle of 20 to the horizontal. (a) Determine the minimum velocity of the serve such that the ball will just clear...
-
9.50. Dipping low ** A top with I = 3/3 floats in outer space and initially spins around its x3 axis with angular speed w3. You apply a strike at the bottom point, directed into the page, as shown in...
-
Which reducing agent, LiAlH4 or NaBH4, would you use to carry out the following practice problem 12.3 transformations? (a) (b) (c) (d) (e) HO HO HO OMe OMe OH
-
Which of the following streaming TV devices does not involve use of a remote controller? A) Google Chromecast B) Apple TV C) Amazon Fire TV D) Roku
-
Convert 6.0 feet per second to meters per second.
-
Compute the pressure produced in the oil in a closed cylinder by a piston exerting a force of 2500 lb on the enclosed oil. The piston has a diameter of 3.00 in.
-
A hydraulic cylinder must be able to exert a force of 8700 lb. The piston diameter is 1.50 in. Compute the required pressure in the oil.
-
why would an auditor want to complete dual-purpose tests? what procedure can be put into place to help prevent fraud? List 4 procedures.
-
Based on the following information, calculate sustainable growth rate for Groot, Inc.: Profit margin= 7.1% Total asset turnover = 1.90 Total debt ratio = .45 Payout ratio = 20% What is the ROA here?
-
Consider the following: a call option on a stock has strike price $100, premium of $5 and the current price of the underlying stock is $100. If you buy the call option today, what is your holding...

Study smarter with the SolutionInn App


