Identify the kinds of intermolecular forces (London dispersion, dipole-dipole, hydrogen bonding) that are important in each of
Question:
Identify the kinds of intermolecular forces (London dispersion, dipole-dipole, hydrogen bonding) that are important in each of the following substances.
(a) Propane (C3H8)
(b) Ethylene glycol [HO(CH2)2OH]
(c) Cyclohexane (C6H12)
(d) Phosphine oxide (PH3O)
(e) Nitrogen monoxide (NO)
(f) Hydroxylamine (NH2OH)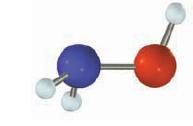
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





