Identify the orbitals that overlap to form the bonds in HCN. HIS- C 5/3 C C
Question:
Identify the orbitals that overlap to form the bonds in HCN.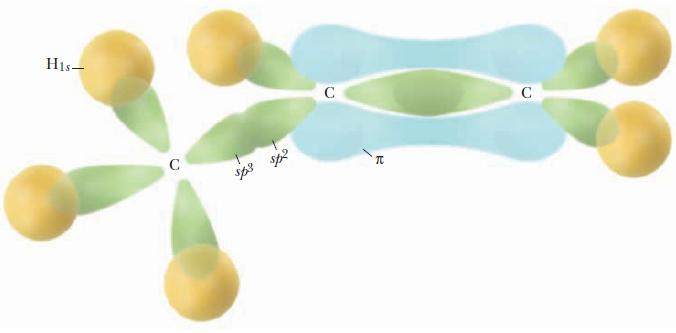
Transcribed Image Text:
HIS- C 5/3 C C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
The sphybridized carbon atom forms two bonds one by overl...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Draw the two resonance structures that describe the bonding in the acetate ion. What is the hybridization of the carbon atom of the CO 2 group? Select one of the two resonance structures and...
-
Classify each of the labeled bonds in the following structure in terms of the bond type ( or ) and the component orbitals that overlap to form the bond. (For example, the carbon--carbon bond in...
-
Classify each of the labeled bonds in the following structure in terms of the bond type ( or ) and the component orbitals that overlap to form the bond. (For example, the carboncarbon bond in ethane...
-
In the figure below, a square of edge lengths is formed by four spheres of masses, m, M, m3, and m4. What is the x component and the y component of the net gravitational force from them on a central...
-
The harmonic decomposition problem considered by Pisarenko can be expressed as the solution to the equation. The solution for a can be obtained by minimizing the quadratic form a*l?yya subject to the...
-
If you bought a share of stock, what would you expect to receive, when would you expect to receive it, and would you be certain that your expectations would be met?
-
This exercise is a very simple version of a model of the bid-ask spread presented by Stoll (1978). Consider an individual with constant absolute risk aversion . Assume w and x are joint normally...
-
The Sea Wharf Restaurant would like to determine the best way to allocate a monthly advertising budget of $1000 between newspaper advertising and radio advertising. Management decided that at least...
-
Based on the segment margin report below which region (central, east coast, international, midwest, northeast, northwest and south) should be dropped or restructured and why? Question 6: Segment...
-
Identify the hybridization of the central atom and the orbitals used for each bond in IF 5 . Strategy The steric number, determined from the Lewis structure, defines the hybrid orbitals needed for a...
-
Describe the bonds in propylene, CH 3 CH=CH 2 . Identify the hybridization of each central atom and the type ( or ) of each bond. Strategy Use the steric numbers determined from the Lewis structure...
-
Reconsider the previous exercise. Conduct a theory-based analysis. Report the test statistic and p-value and summarize your conclusions. Also, compute 95% confidence intervals as a follow up,...
-
A carload of Hg-ore containing grains of cinnabar (86%Hg by mass; density = 8.19 g/cm3) and grains of basalt (containing no Hg; density=2.84 g/cm3) is to be sampled and analyzed for mercury. The...
-
CMS reviews acute IPPS and long-term care hospital (LTCH) records for payment purposes. Documentation and coding assignment must be accurate and specific. CMS contracts with Medicare Administrative...
-
Problem 2. x3+2x+1 f(x) = = 5-x 8H xx (4 points) Without graphing the function, find the limits lim f(x) and lim f(x) analyt- ically and show your work. Specify if the limits are - or +. (1 point)...
-
For change management, answer the following questions in detail, citing some industry examples: 1. What would you do if your manager requested you change your way of working on a project? 2. What do...
-
1.Sony has just released a new CD recording (okay, not new because we don't buy CDS) but anyway.Here is some cost and price information: CD Disc and Packaging (material and labor) $1.75/CD...
-
Sketch a curve that would describe the expected behavior of phosphofructokinase activity as a function of the adenylate energy charge. Adenylate energy charge
-
Ball bearings are widely used in industrial applications. You work for an industrial food machinery manufacturer and your role is to design the driveshaft assembly on a new type of equipment that...
-
Determine the shear strain γ xy at corners A and B if the plate distorts as shown by the dashed lines. 5 mm, 2 mm 4 mm IB 2 mm 300 mm T2 mm -X- 400 mm 3 mm
-
Determine the shear strain γ xy at corners D and C if the plate distorts as shown by the dashed lines. 5 mm, 2 mm 4 mm IB 2 mm 300 mm T2 mm 400 mm- 3 mm
-
The pipe with two rigid caps attached to its ends is subjected to an axial force P. If the pipe is made from a material having a modulus of elasticity E and Poissons ratio v, determine the change in...
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


