Identify two homonuclear diatomic molecules or ions with each of the following molecular orbital electron configurations. Are
Question:
Identify two homonuclear diatomic molecules or ions with each of the following molecular orbital electron configurations. Are these species stable?
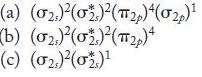
Transcribed Image Text:
(a) (02)(02)(2)(02) (b) (02)(0)(12)4 (c) (0)(0)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The image you provided seems to show three different molecular orbital MO electron configurations fo...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Identify two homonuclear diatomic molecules or ions with each of the following molecular orbital electron configurations. Are these species stable? (a) (0)(0)(2)(2p) (p) (0)(o)(2) 4 (02) (c) (0)(0)...
-
Coca-Cola Company and PepsiCo are two industry leaders in the carbonated soft drink industry. They are strong rivals of each other in the beverage market of the world. From 1975 to 1990s, both the...
-
Today in the spot market $1 = 1.82 Swiss francs and $1 = 130 Japanese yen. In the 90-day forward market, $1 = 1.84 Swiss francs and $1 = 127 Japanese yen. Assume that interest rate parity holds...
-
List all types of bonding present in the compound CaCO3. List all types of bonding present in the compound CaCO3. I. ionic bond II. polar covalent bond III. nonpolar covalent bond A. I only B. II...
-
Calculating Liquidity Ratios SDJ, Inc., has net working capital of $l,570 current liabilities of $4,380, and inventory of $1,875. What is the current ratio? What is the quick ratio?
-
The following T-accounts represent manufacturing cost flows for Lincoln Manufacturing Company for the year 2012. Required: 1. Identify the following amounts for 2012 from Lincoln's T-accounts: a....
-
Review, briefly, a range of performance initiatives? lo1
-
1. Does the national Do Not Call Registry adversely affect the way that business is conducted in this country? If so, how? Should Congress enact a Do Not Spam law? Why or why not? 2. If 90 percent of...
-
Note. Assume that the firm will always be able to utilice its fal interest tak shield. NiChys afiertan WACC is (Round to two decincal placen)
-
Use the molecular orbital diagram in Figure 10.40 to predict which species in each pair has the stronger bond. (a) F 2 or F 2 (b) O 2 or O + 2 (c) C 2+ 2 or C 2 Figure 10.40 Energy Atomic orbitals...
-
Identify the hybridization of the central atom that has the bonded-atom lone-pair arrangement of (a) A tetrahedron. (b) A trigonal bipyramid. (c) An octahedron.
-
Suppose a piece of Styrofoam, p = 180 kg/m3, is held completely submerged in water (Fig. 14.46). (a) What is the tension in the cord? Find this using Archimedes's principle. (b) Use P = Po + pgh to...
-
Sketch plane / intersecting plane K. Then draw a line & in plane J that intersects plane Kat a single point. A X C B D E
-
Use a graphing utility to verify any five of the graphs that you drew by hand in Exercises 126. Data from exercise 1-26 1. x + 2y = 8 3. x2y> 10 2. 3x6y 12 4. 2xy > 4
-
The following information pertains to Porter Company for 2011. Ending inventory consisted of 30 units. Porter sold 320 units at \(\$ 30\) each. All purchases and sales were made with cash. Required...
-
In Problems 7780, use a numerical integration routine on a graphing calculator to find the area bounded by the graphs of the indicated equations over the given interval (when stated). Compute answers...
-
Solar Heating, Inc., had the following transactions for 2011: Required a. Determine the quantity and dollar amount of inventory at the end of the year, assuming Solar Heating Inc. uses the FIFO cost...
-
A 0.25-mm-diameter open tubular gas chromatography column is coated with stationary phase that is 0.25 m thick. The diffusion coefficient for a compound with a retention factor k = 10 is Dm = 1.0 ...
-
Refer to the table to answer the following questions. Year Nominal GDP (in billions) Total Federal Spending (in billions) Real GDP (in billions) Real Federal Spending (in billions) 2000 9,817 578...
-
The beam is made from three boards nailed together as shown. If the moment acting on the cross section is M = 600 N m, determine the resultant force the bending stress produces on the top board. 25...
-
If the built-up beam is subjected to an internal moment of M = 75 kN m, determine the maximum tensile and compressive stress acting in the beam. 150 mm 20 mm- 50 mm 150 mm 10 mm 10 mm 300 mm
-
If the built-up beam is subjected to an internal moment of M = 75 kN m, determine the amount of this internal moment resisted by plate A.
-
Q1) The equity of Washington Ltd at 1 July 2020 consisted of: Share capital 500 000 A ordinary shares fully paid $1 500 000 400 000 B ordinary shares issued for $2 and paid to $1.50 600 000 General...
-
out The following information relates to Questions 1 to 2. The management accountant of a furniture manufacturer is developing a standard for the labour cost of one massage chair. When operating at...
-
Exercise 10-8 Utilization of a constrained Resource [LO10-5, L010-6] Barlow Company manufactures three products: A, B, and C. The selling price, variable costs, and contribution margin for one unit...

Study smarter with the SolutionInn App


