Use the molecular orbital diagram in Figure 10.40 to predict which species in each pair has the
Question:
Use the molecular orbital diagram in Figure 10.40 to predict which species in each pair has the stronger bond.
(a) F2 or F−2
(b) O−2 or O+2
(c) C2+2 or C2
Figure 10.40 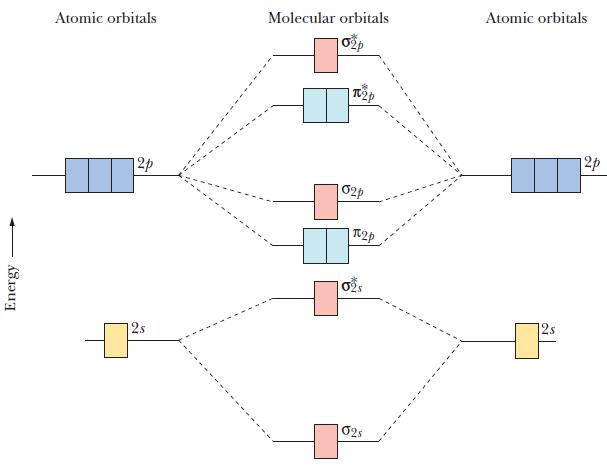
Transcribed Image Text:
Energy Atomic orbitals 12p 2s Molecular orbitals + Tp 02p 2p 02s Atomic orbitals 12p
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The molecular orbital MO diagram youve provided is a generic one for diatomic molecules of second pe...View the full answer

Answered By

Joemar Canciller
I teach mathematics to students because I love to share what I have in this field.
I also want to see the students to love math and be fearless in this field.
I've been tutoring these past 2 years and I would like to continue what I've been doing.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the molecular orbital diagram in Figure 10.40 to predict which species in each pair has the stronger bond. (a) B 2 or B 2 (b) C 2 or C + 2 (c) O 2+ 2 or O 2 Figure 10.40 Energy Atomic...
-
Which species of each pair has the greater De? (a) Li2 or Li+2 ; (b) C2 or C+2; (c) O2 or O+2; (d) F2 or F+2.
-
(a) The allyl anion has an unshared electron pair on the allylic carbon: This anion has two more electrons than the allyl cation. Use the molecular orbital diagram in Fig. 15.14b to decide which...
-
This exercise investigates the way in which conditional independence relationships affect the amount of information needed for probabilistic calculations. a. Suppose we wish to calculate P (he1, e2)...
-
Calculating the Average Collection Period Pujols Lumber Yard has a current accounts receivable balance of $387,615. Credit sales for the year just ended were $2,945,600. What is the receivables...
-
Why do managers need accurate product cost information?
-
What/who is the source of each initiative? lo1
-
Elliot Karlin is a 35-year-old bank executive who has just inherited a large sum of money. Having spent several years in the banks investments department, hes well aware of the concept of duration...
-
On April 1, 2023, ET Inc. has available for issue $438,000 bonds due in four years. Interest at the rate of 4.0% is to be paid quarterly. Calculate the issue price if the market interest rate is: (Do...
-
Which species, O 2 or O 2 , has the higher bond order? Explain your answer.
-
Identify two homonuclear diatomic molecules or ions with each of the following molecular orbital electron configurations. Are these species stable? (a) (0)(0)(2)(2p) (p) (0)(o)(2) 4 (02) (c) (0)(0)...
-
What costs and benefits are there to offering trade credit? How can a small firm ensure that it controls the costs?
-
The following post-closing trial balance was drawn from the accounts of Spruce Timber Co. as of December 31, 2011. Transactions for 2012 1. Acquired an additional \(\$ 10,000\) cash from the issue of...
-
Bankers Trust (BT) was one of the most powerful and profitable banks in the world in the early 1990s. Under the stewardship of chairman Charles Sanford Jr., it had transformed itself from a staid...
-
Hammond Inc. experienced the following transactions for 2011, its first year of operations: 1. Issued common stock for \(\$ 80,000\) cash. CHECK FIGURES b. Net Income: \(\$ 62,520\) Total Assets:...
-
Following are the current prices and last years prices of a gallon of regular gas at a sample of 14 gas stations. Can you conclude that the median price is different now from what it was a year ago?...
-
A sample of nine men participated in a regular exercise program at a local gym. They were weighed both before and after the program. The results were as follows. Can you conclude that the median...
-
The distribution coefficient for extraction of a metal complex from aqueous to organic solvents is D = [total metal] org /[total metal] aq . Give physical reasons why and Ka appear in the numerator...
-
Refer to the information from Exercise 22-19. Use the information to determine the (1) Weighted average contribution margin , (2) Break-even point in units, and (3) Number of units of each product...
-
Draw the shear and moment diagrams for the simply supported beam. 10 kN 10 kN 15 kN-m -2 m -2 m 2 m
-
Draw the shear and moment diagrams for the double overhanging beam. 400 lb 400 Ib 200 lb/ft -3 ft 6 ft -3 ft-
-
The beam is used to support a uniform load along CD due to the 6-kN weight of the crate. Also, the reaction at the bearing support B can be assumed uniformly distributed along its width. Draw the...
-
The number of hours studied and the scores that students earned are shown. Creating a scatter chart, which statement is true regarding the relationship between the hours of study and scores earned....
-
Five samples of 12 each were extracted from a population. Based on the central limit theorem, what is the best estimate of the SD of the population? A B C D E 270 230 290 238 315 303 274 270 246 244...
-
In a national test, the mean score was 1575 and the standard deviation was 85. What % of the students earned a score between1500 and 1600 if the scores were Normally distributed?

Study smarter with the SolutionInn App


