In 1926, Hinshelwood and Green studied the reaction of nitrogen monoxide and hydrogen. (a) What is the
Question:
In 1926, Hinshelwood and Green studied the reaction of nitrogen monoxide and hydrogen.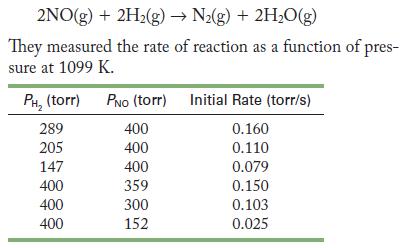
(a) What is the rate law for the reaction?
(b) Use the data from the first experiment to calculate the rate constant at 1099 K, using torr as the concentration unit.
(c) The relative rate of the reaction changed with temperature, as the following data show.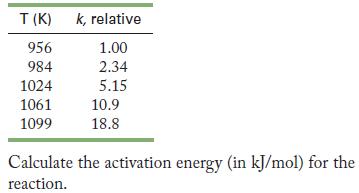
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





