Nitrogen monoxide reacts with ozone. NO(g) + O3(g) NO(g) + O(g) Two mechanisms have been proposed: Mechanism
Question:
Nitrogen monoxide reacts with ozone.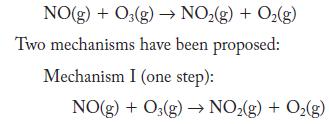
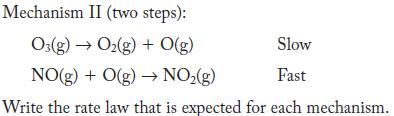
Transcribed Image Text:
NO(g) + O3(g) NO(g) + O(g) Two mechanisms have been proposed: Mechanism I (one step): NO(g) + O3(g) NO(g) + O(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The rate law for a chemical reaction describes how the rate of reaction depends on the concentration ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Nitrogen monoxide reacts with oxygen to give nitrogen dioxide. 2NO(g)+O2(g) 2NO2(g) The rate law is [NO]/ t = k[NO]2[O2], where the rate constant is 1.16 105 L2/(mol2s) at 339oC. A vessel contains...
-
Nitrogen monoxide reacts with hydrogen as follows: 2NO(g)+ H2(g) N2O(g) + H2O(g) The rate law is [H2]/ t = k[NO]2[H2], where k is 1.10 107 L2/(mol2s) at 826oC. A vessel contains NO and H2 at...
-
Consider the reaction of ozone and nitrogen monoxide to form nitrogen dioxide and oxygen. Which of the following orientations for the collision between ozone and nitrogen monoxide could perhaps lead...
-
Four of Wands, LLC generated $255,000 in sales during January 2022. Of this amount, 25% was for cash. The remaining 75% of sales were made on account. The February 2022 sales on account were...
-
Find I0 and I1 in the circuit infigure. 5 mA 4 mA 2 mA 3 mA ww (+1)
-
Carl Foster, a trainee at an investment banking firm, is trying to get an idea of what real rate of return investors are expecting in todays marketplace. He has looked up the rate paid on 3-month...
-
Explain the basics of a performance evaluation system. LO.1
-
What type of research studies lend themselves to the use of e-mail for survey research? What are the advantages and disadvantages of using e-mail?
-
The Assistant Accountant of Sydney Ltd is reviewing wages and salaries expenses and notes the following: A. pays its salaries fortnightly in arrears. The next pay day is Thursday 2 July. The...
-
The gas-phase reaction of nitrogen monoxide with chlorine proceeds to form nitrosyl chloride. 2NO(g) + Cl(g) 2NOC1(g) rate = k[NO][C1] Evaluate the following proposed mechanism to determine whether...
-
In 1926, Hinshelwood and Green studied the reaction of nitrogen monoxide and hydrogen. (a) What is the rate law for the reaction? (b)Use the data from the first experiment to calculate the rate...
-
Convert 2017 to a numeral in the base indicated. 6
-
1) What are the benefits of home-based working for the company and the employees? 2) What are the challenges in performance management in working from home? 3) What is the right mix of office-based...
-
This assignment is focused on project selection and the underlying factors used to make this determination. You will need to use the readings/videos, the previous learning modules, along with some...
-
1. While improper framing could affect the information we have on sark attacks, I think our decisions come down to "anchoring and adjustment". Because the information we received from the media was...
-
For each of the scenarios in the following table, indicate the most likely reason for the difference in earnings. Scenario Differences in Human Capital Compensating Differential Differences in...
-
All organizations whether it is the government, a private business or small businessman require planning. To turn their dreams of increase in sale, earning high profit and getting success in business...
-
Draw the structure of the product from the following reaction (formed during a synthesis of one of the endiandric acids by K. C. Nicolaou): MeO2C osi(t-Bu)Phe toluene, 110C
-
Cobb Manufacturing Company uses a process cost system and average costing. The following production data is for the month of June 2011. Production Costs Work in process, beginning of the month:...
-
Sketch the influence line for (a) The moment at E, (b) The reaction at C, and (c) The shear at E. In each case, indicate on a sketch of the beam where a uniform distributed live load should be placed...
-
Draw the influence line for the reaction at C. Plot the numerical values every 5 ft. EI is constant. B -15 ft- -15 ft-
-
Draw the influence line for the shear at C. Plot numerical values every 1.5 m. Assume A is fixed and the support at Bis a roller. EI is constant. SA B 3 m 3 m
-
ABC Company engaged in the following transaction in October 2 0 1 7 Oct 7 Sold Merchandise on credit to L Barrett $ 6 0 0 0 8 Purchased merchandise on credit from Bennett Company $ 1 2 , 0 0 0 . 9...
-
Lime Corporation, with E & P of $500,000, distributes land (worth $300,000, adjusted basis of $350,000) to Harry, its sole shareholder. The land is subject to a liability of $120,000, which Harry...
-
A comic store began operations in 2018 and, although it is incorporated as a limited liability company, it decided to be taxed as a corporation. In its first year, the comic store broke even. In...

Study smarter with the SolutionInn App


