K c at 137 C is 4.42 for
Question:
Kc at 137 °C is 4.42 for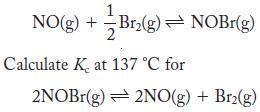
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
K ...View the full answer

Answered By

Muhammad adeel
I am a professional Process/Mechanical engineer having a vast 7 years experience in process industry as well as in academic studies as a instructor. Also equipped with Nebosh IGC and lead auditor (certified).
Having worked at top notch engineering firms, i possess abilities such as designing process equipment, maintaining data sheets, working on projects, technical biddings, designing PFD and PID's etc.
Having worked as an instructor in different engineering institutes and have been involved in different engineering resrearch projects such as refinery equipment designing, thermodynamics, fluid dynamics, chemistry, rotary equipment etc
I can assure a good job within your budget and time deadline
4.90+
52+ Reviews
60+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1 and 2. On September 1, Irene opened a retail store that specializes in sports car...
-
Draw a plausible mechanism for each of the following transformations: (a) (b) [TSOH] MENH2 -H20
-
How could you use 1H NMR, 13C NMR, and IR spectroscopy to help you distinguish between the followingstructures? "CH 3-Methyl-2-cyclohexenone 3-Cyclopentenyl methyl ketone
-
The Fed promotes secrecy by not releasing FOMC directives to Congress or the public immediately. Discuss the pros and cons of this policy.
-
E19-1 Listed below are several terms relating to the functions of managers and the differences between financial and management accounting. Complete the following statements with one of these terms....
-
A chocolate maker has contracted to operate a small candy counter in a fashionable store. To start with, the selection of offerings will be intentionally limited. The counter will offer a regular mix...
-
1 of 3 Required information (The following information applies to the questions displayed below.) Jeremy earned $100,500 in salary and $6,250 in interest income during the year. Jeremy's employer...
-
The state table for a 3-bit twisted ring counter is given in Table 4-15. This circuit has no inputs, and its outputs are the uncomplemented outputs of the lip- lops. Since it has no inputs, it simply...
-
Identify the conjugate pairs. Strategy Identify the species that differ by a protonthese are the conjugate pairs. The acid form has the proton; the base form lacks the proton.
-
The set of Mike and Carol's children on the TV show, The Brady Bunch. For the following exercises, represent each set using the method of your choice.
-
1. A corn farmer has observed the following distribution for the number of ears of corn per cornstalk. Ears of Corn Probability 1 2 3 4 .3 .4 .2 .1 Part A: How many ears of corn does he expect on...
-
1. A mass m on a vertical spring with force constant k has an amplitude of A. Using the top of the motion as the origin for both gravitational potential energy and spring potential energy: (a) Find...
-
2. Consider the PDE Utt - Uxx + Ut - Ux = 0 (1) for < < and 0
-
On April 1, 2024, Chardonnay pays an insurance company $12,480 for a two- year fire insurance policy. The entire $12,480 is debited to Prepaid Insurance at the time of the purchase. Record the...
-
Which retailer(s) should represent and sell your product?Why?In terms of their range of distribution coverage, is your retailer intensive, selective and exclusive? Why is this aspect important to...
-
The molecule n-octylglucoside, shown here, is widely used in biochemical research as a nonionic detergent for "solubilizing" large hydrophobic protein molecules. What characteristics of this molecule...
-
United Business Forms capital structure is as follows: Debt ............................................ 35% Preferred stock ........................... 15 Common equity .......................... 50...
-
Which of the following expressions correspond to traveling waves? For each of those, what is the speed of the wave? The quantities , b, and c are positive constants. (a) (z, t) = (z - bt) 2 (b) (x,...
-
The profile of a transverse harmonic wave, traveling at 1.2 m/s on a string, is given by y = (0.02 m) sin (157 m -1 ) x Determine its amplitude, wavelength, frequency, and period.
-
Consider the plane electromagnetic wave in vacuum (in SI units) given by the expressions E x = 0, E y = 2 cos [2 10 14 (t - x/c) + /2], and E z = 0. (a) What are the frequency, wavelength, direction...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?
-
NEED THIS EXCEL TABLE ASAP PLEASE!!!! Presupuesto Operacional y C lculo del COGS Ventas Proyectadas: Ventas Proyectadas: $ 4 5 0 , 0 0 0 Precio por unidad: $ 4 5 0 Unidades vendidas: 4 5 0 , 0 0 0 4...
-
The wash sale rules apply to disallow a loss on a sale of securities_______? Only when the taxpayer acquires substantially identical securities within 30 days before the sale Only when the taxpayer...

Study smarter with the SolutionInn App


