Nitrogen dioxide reacts with carbon monoxide to form carbon dioxide and nitrogen monoxide. NO(g) + CO(g) CO(g)
Question:
Nitrogen dioxide reacts with carbon monoxide to form carbon dioxide and nitrogen monoxide.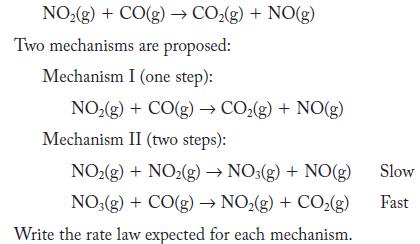
Transcribed Image Text:
NO(g) + CO(g) CO(g) + NO(g) Two mechanisms are proposed: Mechanism I (one step): NO(g) + CO(g) CO(g) + NO(g) Mechanism II (two steps): NO(g) + NO2(g) NO3(g) + NO(g) NO3(g) + CO(g) NO(g) + CO(g) Write the rate law expected for each mechanism. Slow Fast
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
by mechani...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Nitrogen dioxide reacts with carbon monoxide to form carbon dioxide and nitrogen monoxide. The overall stoichiometry is Evaluate the following mechanism to determine whether it is consistent with...
-
The mechanism for the gas-phase reaction of nitrogen dioxide with carbon monoxide to form nitric oxide and carbon dioxide is thought to be Write the rate law expected for this mechanism. What is the...
-
Nitrogen dioxide reacts with carbon monoxide by the overall equation NO2(g)+ CO(g) NO(g) + CO2(g) At a particular temperature, the reaction is second order in NO2 and zero order in CO. The rate...
-
A school district comprises 24 schools. The numbers of students in each of the schools are as follows: The district wants to implement an experimental teaching and learning model for approximately...
-
Find Vac in the circuit infigure: 4 V 3Vx 12 v+ Vx = 2V
-
You plan to retire in exactly 20 years. Your goal is to create a fund that will allow you to receive $20,000 at the end of each year for the 30 years between retirement and death (a psychic told you...
-
Have I visited the organizations Web site to learn more about the company, and done an Internet search to read a few articles about the company? LO.1
-
A mass balance for a chemical in a completely mixed reactor can be written as Vdc/dt = F Qc kVc2 where V = volume (12 m3), c = concentration (g/m3), F = feed rate (175 g/min), Q = flow rate (1...
-
Stock Options On January 1, Y6, the shareholders of Aylmer approved a plan that grants options to the companys executives to purchase 10,000 of the companys common shares each. The options are...
-
The decomposition of hydrogen peroxide is catalyzed by horseradish peroxidase, an enzyme isolated from the vegetable. The hydrogen peroxide concentration is measured as a function of time to produce...
-
Write the rate law and the molecularity for each of the following elementary reactions. (a) NO+NOCl NO + NOCI (b) NO + SO NO + SO3 (c) NO4 2NO
-
Two masses are connected by springs as shown in the diagram. Both springs have the same spring constant, and the end of the first spring is fixed. If x1 and x2 represent the displacements from the...
-
How can you filter on a particular data field in Cognos Analytics? 0 / 1 point Type in the name of the field in the 'Filters' area at the top of the page. Drag the data field to the 'All tabs' area...
-
Project management is fundamental to project success and includes a number of critical success factors including support of top management, use of effective communication channels and rapid feedback,...
-
BIO 189: what are some of the ethical issues regarding his results? why were his results rejected by the scientific community? choose two components below that were either flawed or completely absent...
-
REQUIRED: Prepare Cost of Production per department. Problem 5 ABM Company uses two departments to produce a product. The following data were taken from the books for the month of January, 2019....
-
Can you describe how Toyota responds to changes in market fluctuations, and plot the supply and demand curves indicating managerial economic principles (i.e. price ceilings/floors, shortage/surplus,...
-
Azulene has an appreciable dipole moment. Write resonance structures for azulene that explain this dipole moment and that help explain its aromaticity.
-
6 (a) Briefly develop a mathematical model of the behaviour of a copper-twisted pair cable (b) Derive the magnetic energy from: w given that: K + w, where the - - k symbols have their usual meaning...
-
Determine the vertical displacement of joint C of the truss. Each member has a cross-sectional area of A = 300 mm 2 . E = 200. Using Castiglianos theorem. 3 m 4 m 4 m 4 m 3 kN 3 kN 4 kN
-
Determine the vertical displacement of joint C of the truss. Each member has a cross-sectional area of A = 300 mm 2 . E = 200. Use the method of virtual work. 3 m 4 m 4 m 4 m 3 kN 3 kN 4 kN
-
Determine the horizontal displacement of joint D. Assume the members are pin connected at their end points. AE is constant. Using Castiglianos theorem. 8 m 2k D. 6 ft 3k 6 ft
-
question 1- You borrow a simple loan of SR 500,000, interest rate is 20%, it matures in one year. what's the yied to maturity? question 2- calculate_i for One-Year Discount Bond with price(p) =...
-
Taste of Muscat is a reputed chain of restaurants operating in Oman. Assume You are working as a management accountant for this restaurant chain which is specialized in all types of Arabic food. Your...
-
Industry Current Year Minus 1 Current Year Minus 2 Company: Air Products and Chemicals, Inc. (APD) Stock Price: 306.72 USD Shares Outstanding: 220.89 M Financial Ratios Most Current Year Current...

Study smarter with the SolutionInn App


