Partial information is given in each column in the following table. Fill in the blank spaces. Symbol
Question:
Partial information is given in each column in the following table. Fill in the blank spaces.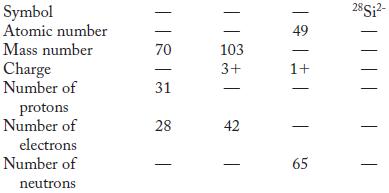
Transcribed Image Text:
Symbol Atomic number Mass number Charge Number of protons Number of electrons Number of neutrons. 70 31 28 | || 103 3+ - - - 49 1+ 65 28Si²-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Symbol Atomic number Mass number Charge N...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The following table summarizes the facts of five independent cases (labeled a through e) of U.S. companies engaging in credit transactions with foreign corporations while the foreign exchange rate is...
-
Start of Payroll Project 7-3a October 9, 20-- No. 1 The first payroll in October covered the two workweeks that ended on September 26 and October 3. This payroll transaction has been entered for you...
-
Given the partial information in each column of the following table, fill in the blanks. Symbol Atomic number Mass number Charge Number of protons Number of electrons Number of neutrons 11 10 12...
-
Four different processes for baking Oreo cookies are considered for the 2008 season. The cookies produced by each process are evaluated in terms of their overall quality. Since the cookies sometimes...
-
The 2010 accounting records of Verlander Transport reveal these transactions and events. InstructionsPrepare the cash flows from operating activities section using the direct method. (Not all of the...
-
Use Stokes Theorem to evaluate C F dr. In each case C is oriented counterclockwise as viewed from above, unless otherwise stated. F(x, y, z) = x 2 y i + x 3 j + e z tan 1 z k, C is the curve with...
-
What conditions are required for valid inferences about the bs in simple linear regression? LO9
-
Niner Bikes, as discussed in the chapter opener, uses a costing system with standard costs for direct materials, direct labor, and overhead costs. Two comments frequently are mentioned in relation to...
-
Part 1 of 3 Required information Assume Down, Inc., was organized on May 1 to compete with Despair, Inc.-a company that sells de-motivational posters and office products. Down, Inc., encountered the...
-
Write the formula of each of the following compounds, and indicate whether each is ionic or molecular. (a) Calcium phosphate (b) Germanium dioxide (c) Iron(III) sulfate (d) Phosphorus tribromide
-
Name each of the following compounds, and indicate whether each is ionic or molecular. (a) NO (b) Y 2 (SO 4 ) 3 (c) Na 2 O (d) NBr 3
-
Picture being an employee of Delta during the period where the company transitioned into and out ofbankruptcy. What motivational implications would that experience have, and how long would they last?
-
Lucy is using a one-sample test based on a simple random sample of size = 24 to test the null hypothesis = 23.000 cm against the alternative hypothesis < 23.000 cm. The sample has mean 22.917 cm and...
-
A motorcyclist of mass 60 kg rides a bike of mass 40 kg. As she sets off from the lights, the forward force on the bike is 200N. Assuming the resultant force on the bike remains constant, calculate...
-
A load downward load P = 400 N is applied at B. It is supported by two truss members with member BA at an angle of 0 = 45 from horizontal and member BC at an 01 = angle of 02 25 from vertical....
-
Gross profit, defined as Net sales less Cost of products sold increased by $279 million in 2017 from 2016 and decreased by $2 million in 2016 from 2015. As a percent of sales, gross profit was 38.8%...
-
An electro-magnetic shield is to be made of galvanized steel with conductivity = 1.74 x 106 S/m, and magnetic permeability HR = 80. The thickness of cold rolled steel is in the following table. Gauge...
-
The general ledger of the Pueblo Company contained the following accounts, among others, on January 1: Finished Goods, $15,000; Work in Process, $30,000; Materials, $25,000. During January the...
-
From 1970 to 1990, Sri Lanka's population grew by approximately 2.2 million persons every five years. The population in 1970 was 12.2 million people.What is the best formula for P, Sri Lanka's...
-
A compound with molecular formula C 5 H 10 O 2 has the following NMR spectrum. Determine the number of protons giving rise to each signal. Proton NMR lle 2.0 1.5 0.5 ppm 4.5 4.0 33.2 3.5 3.0 2.5 1.0...
-
A compound with molecular formula C 10 H 10 O has the following NMR spectrum. Determine the number of protons giving rise to each signal. Proton NMR 10 3 1 ppm 4 87.1| 18.7 Integration Values 17.1...
-
A compound with molecular formula C 4 H 6 O 2 has the following NMR spectrum. Determine the number of protons giving rise to each signal. Proton NMR 25 2.0 2.0 1.5 ppm 3.0 Integration Values 5.0 4.5...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


