Toxic nitrogen monoxide gas can be prepared in the laboratory by carefully mixing a dilute sulfuric acid
Question:
Toxic nitrogen monoxide gas can be prepared in the laboratory by carefully mixing a dilute sulfuric acid with an aqueous solution of sodium nitrite, as the following equation shows. What volume of 1.22 M sulfuric acid (assume excess sodium nitrite) is needed to prepare 2.44 g NO?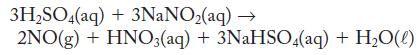
Transcribed Image Text:
3H₂SO4(aq) + 3NaNO₂(aq) → 2NO(g) + HNO3(aq) + 3NaHSO4(aq) + H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)

Answered By

Deepak Pal
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students. Areas of interest: Business, accounting, Project management, sociology, technology, computers, English, linguistics, media, philosophy, political science, statistics, data science, Excel, psychology, art, history, health education, gender studies, cultural studies, ethics, religion. I am also decent with math(s) & Programming. If you have a project you think I can take on, please feel welcome to invite me, and I'm going to check it out!
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
Refer to the Integrative Example on page 140. If 138 g Na 2 CO 3 in 1.42 L of aqueous solution is treated with an excess of NO(g) and O 2 (g), what is the molarity of the NaNO 2 (aq) solution that...
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
For this project, you must select an employer organization and research the organizations employee benefits package (plan). After you research the organizations employee benefits package, collect...
-
Plot the payoff diagrams fur the following instruments: (a) A caplet with cap rate Rcap = 6.75% written on 3-wonth Libor Lt, that is about to expire. (b) A forward contract written on a default-free...
-
Using the appropriate interest table, provide the solution to each of the following four questions by computing the unknowns. Click here to view factor tables What is the amount of the payments that...
-
What needs are Conway, Wang, and Sanchez attempting to satisfy? LO.1
-
Stewart Beauf is a self-employed surfboard maker in 2018. His Schedule C net income is $125,003 for the year. He also has a part-time job and earns $15,600 in wages subject to FICA taxes. Calculate...
-
15-22 Allocating costs of support divisions, step-down and direct methods. (LO 3) The Bow River Company has prepared division overhead budgets for budgeted-volume levels before allocations as...
-
You recently accepted the controller position for Java the Hut, a regional coffee chain. The owner informs you that a complete financial statement package will be required for a new bank loan...
-
Although silver chloride is insoluble in water, adding ammonia to a mixture of water and silver chloride causes the silver ions to dissolve because of the formation of [Ag(NH 3 ) 2 ]+ ions. What is...
-
Sodium thiosulfate, Na 2 S 2 O 3 , is used in photographic film developing. Th e amount of Na 2 S 2 O 3 in a solution can be determined by a titration with I 2 , according to the following equation:...
-
The structure of the nitro group (-NO2) is usually shown as Experiments show that the two nitrogen-oxygen bonds have the same length of 1.21 Ã. This length is intermediate between 1.36...
-
how could a government or world leader have used ERM to respond to one of the financial, operational, or governance aspects of the covid19 pandemic? include references for further reading.
-
Computing and Interpreting Return on investment Selected operating data for two divisions of Outlook Brewing, Ltd., of Australia are given below: Division Queensland New South Wales Sales: $4,000,000...
-
Consider a parcel of land that contains an even ages stand of trees currently of age in A in t=0. you have to decide how much longer to allow this stand to grow given that when you cut the stand, you...
-
What does the company report for the following accounts for the most current fiscal year:Enter your answer in thousands.a . Cash$fill in the blank 1 1 , 1 5 4 , 8 6 7 b . Short - term investments (...
-
Consider the translational mechanical system with a nonlinear spring shown below. The spring is defined by s(t)=ks(t), where x(t) is the spring length and f(t) the spring force. Nonlinear spring 0000...
-
In what ways can shares be "preferred"? In which ways are they similar to common shares? Different from common shares?
-
If 2 5 9 - k 5 8 = 2 5 8 , what is the value of k?
-
Draw the structure of each of the following compounds. a. (R) -2-Ethoxy-1, 1-dimethylcyclobutane b. Cyclopropyl isopropyl ether
-
Show that the van der Waals and RedlichKwong equations of state reduce to the ideal gas law in the limit of low gas density.
-
An initial step in the biosynthesis of glucose C 6 H 12 O 6 is the carboxylation of pyruvic acid CH 3 COCOOH to form oxaloacetic acid HOOCCOCH2COOH CH 3 COCOOH(s) + CO 2 (g) HOOCCOCH 2 COOH(s) If...
-
Choose two stocks from the same industry to minimize the influence of other confounding factors. You choose the industry that you are relatively more familiar with, and then estimate the implied...
-
why should Undertake research to review reasons for previous profit or loss?
-
A pension fund's liabilities has a PV01 of $200 million. The plan has $100 billion of assets with a weighted average modified duration of 8. The highest duration bond that the plan can invest in has...

Study smarter with the SolutionInn App


