Use the information in Tables 15.6 and 15.8 to calculate K b for the formate ion at
Question:
Use the information in Tables 15.6 and 15.8 to calculate Kb for the formate ion at 25 °C.
Strategy
Because formate ion is the conjugate base of formic acid, use Table 15.6 to find Ka for formic acid; then calculate Kb for the formate ion from the relationship between Ka and Kb.
Table 15.6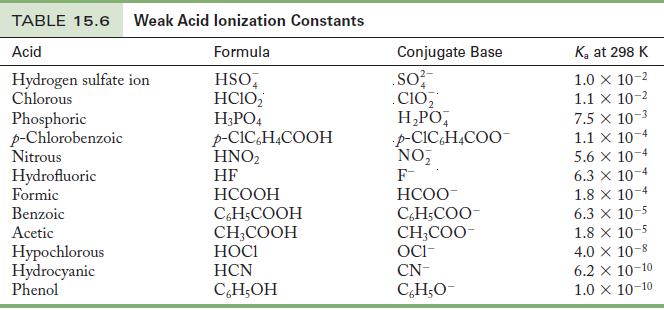
Table 15.8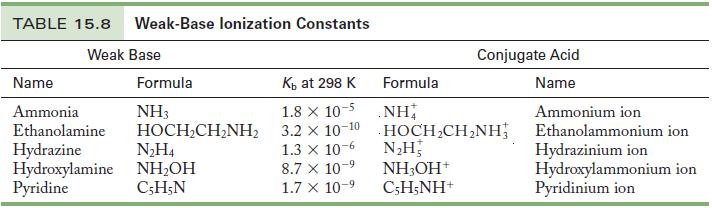
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
KK Kw Kw ...View the full answer

Answered By

Stephen ouma
I have worked with different academic writing companies such as wriredom, writerbay, and Upwork. While working with these companies, I have helped thousands of students achieve their academic dreams. This is what I also intend to do here in SolutionInn
4.90+
19+ Reviews
63+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write the chemical formula for the conjugate base of formic acid, HCOOH and calculate its pK b from the pK a of formic acid (see Table 6C.1). TABLE 6C.1 Acidity Constants at 25 C* K 3.0 X 10-1 2.0 X...
-
Use the information in Table C.1 to determine whether the sample provides evidence of a difference between Servers B and C in the proportion of bills paid with cash. Table C.1 Refer to the dataset...
-
Ant venom contains formic acid (HCOOH; formica is the Latin word for ant). Suppose you are at a pharmaceutical company working on a quick antidote and need to estimate the pH at the stoichiometric...
-
1. Construct a simple pendulum starting with 20 cm length. 2. Hang the pendulum as pictures in one of the two methods shown above. 3. Using the protractor, displace the pendulum 10 degrees from the...
-
Identify the indicated sets of protons as unrelated, homotopic, enantiotopic, ordiastereotopic: (c) (b) (a) - HC- - H3C FCH2
-
If the public expects the Fed to pursue a policy is likely to raise short-term interest rates permanently to 12% but the Fed does not go through with this policy change, what will happen to long-term...
-
DE 19-12 Turn to Exhibit 19-12 (page 767). If direct material purchases were $35,000 rather than $27,000, what would be the cost of direct materials used and the cost of goods manufactured? (Other...
-
After training, Mary Fernandez, a computer technician, had an average observed time for memory-chip tests of 12 seconds. Marys performance rating is 100%. The firm has a personal fatigue and delay...
-
Hello thanks in advance ASAP it will be appraised. USE THIS INFORMATION FOR THE NEXT FEW QUESTIONS: We find the following information for the Welding Department WIP Account of BNSF line. After the...
-
Calculate the pH of household ammonia, which is a 1.44 M aqueous solution of NH 3 . The numerical value of Kb is 1.8 10 -5 at 25 C. Strategy Write the chemical equation, the iCe table, and the...
-
Rank the following bases in order of relative strength, from weakest to strongest: formate ion, cyanide ion, and acetate ion. Strategy This problem can be solved without calculations, because Table...
-
Match each of the key terms with the definition that best fits it. ____________ A diagram that depicts the use cases and actors for a system. Here are the key terms from the appendix. The page where...
-
Context This task requires analysing a network scenario, design the network architecture and recommend IT solutions including ethical, security and sustainability considerations.The purpose of this...
-
What was the Prime Cost Percent for Mandy's BBQ Pit for August? Select one: a. 46.5% b. 73.9% c. 63.4% d. 85%
-
Finding Critical Values and Confidence Intervals. In Exercises 5-8, use the given information to find the number of degrees of freedom, the critical values x? and x*, and the confidence interval...
-
An investor sold 100 shares of ABC stock short at $25 and buys one ABC Jan 30 call @ $5. What is this investor's maximum gain, maximum loss, and breakeven points from this strategy?
-
Jake, Sachs and Brianne own a tour company called Adventure Sports. The partners share profits and losses in a 1:3:4 ratio. After Lengthy Dissagreements among the partners and several unprofitable...
-
A lithium salt used in lubricating grease has the formula LiCnH2n+1O2. The salt is soluble in water to the extent of 0.036 g per 100 g of water at 25 oC. The osmotic pressure of this solution is...
-
A superior criticized a sales manager for selling high-revenue, low-profit items instead of lower-revenue but higher-profit items. The sales manager responded, My income is based on commissions that...
-
Prove that the irradiance of a harmonic EM wave in vacuum is given by and then determine the average rate at which energy is transported per unit area by a plane wave having an amplitude of 15.0 V/m....
-
A nearly cylindrical laserbeam impinges normally on a perfectly absorbing surface. The irradiance of the beam (assuming it to be uniform over its cross section) is 40 W/cm 2 . If the diameter of the...
-
A cloud of locusts having a density of 100 insects per cubic meter is flying north at a rate of 6 m/min. What is the flux density of locusts? That is, how many cross an area of 1 m 2 perpendicular to...
-
nformation pertaining to Noskey Corporation s sales revenue follows: November 2 0 2 1 ( Actual ) December 2 0 2 1 ( Budgeted ) January 2 0 2 2 ( Budgeted ) Cash sales $ 1 0 5 , 0 0 0 $ 1 1 5 , 0 0 0...
-
The management team of Netflix maintains a stable dividend using the Lintner model: Dt+1 = Dt + EPS Target Payout Where Dt (Dt+1) = dividend in the current period t (the next period t + 1) EPSt =...
-
#1 #2 hapter 50 10 D Werences lav Help Required information [The following information applies to the questions displayed below) Archer Company is a wholesaler of custom-built air-conditioning units...

Study smarter with the SolutionInn App


