Use the standard reduction potentials in Table 18.1 to find (a) A reducing agent that will reduce
Question:
Use the standard reduction potentials in Table 18.1 to find
(a) A reducing agent that will reduce Cu2+ but not Pb2+.
(b) An oxidizing agent that will react with Cu but not Fe2+.
(c) A metal ion that can reduce Fe3+ to Fe2+.
Table 18.1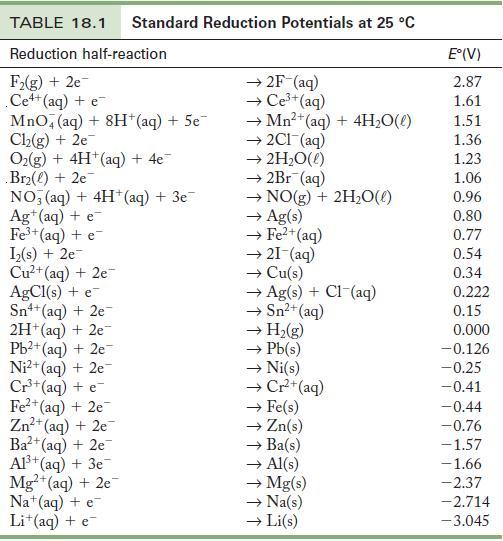
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Keq Ni Cu2 ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write an expression and determine a value for K eq for each voltaic cell in Exercise 18.44 . Exercise 18.44 Use the standard reduction potentials in Table18.1 to find (a) A reducing agent that will...
-
Use the standard reduction potentials in Table 18.1 To find (a) A metal ion that reduces Ni 2+ . (b) A metal ion that can oxidize Cu. (c) A metal ion that is reduced by Cr 2+ but not H 2 . Table 18.1
-
Use the standard reduction potentials to find the equilibrium constant for each of the following reactions at 25°C: (a) (b) (c) Br2(1) + 21-(aq )- 2Br_(aq) + 12(s) 5Fe2 + (aq) +MnO4 (aq ) + 8H +...
-
Examples using activity-based costing generally show that traditional costing systems ________ high-volume, less complex products and ________ low-volume, complex products undercost; overcost...
-
An annular fin of thickness t is used as a radiator to dissipate heat for a space power system. The fin is insulated on the bottom and may be exposed to solar irradiation GS. The fin is coated with a...
-
What are some of the differences between conventional presentations and multimedia presentations with presentation software such as PowerPoint? How would you prepare for each?
-
The Procter & Gamble Company (P&G) The financial statements of P&G are presented in Appendix 5B or can be accessed on the KWW website. Instructions Refer to P&Gs financial statements and the...
-
Using a present value table, your calculator, or a computer program present value function, answer the following questions: Required: a. What is the present value of nine annual cash payments of...
-
Why does a company report comprehensive income?
-
Write an expression and determine a value for K eq for each voltaic cell in Exercise 18.43 . Exercise 18.43 Use the standard reduction potentials in Table18.1 to find (a) A metal ion that reduces Ni...
-
Use the data in Appendix H and assume standard conditions when answering the following questions.
-
An advertisement claims that a particular automobile can "stop on a dime." What net force would actually be necessary to stop a 850-kg automobile traveling initially at 45.0 km/h in a distance equal...
-
a) Discuss whether bike paths can be considered a public good. Now consider a hypothetical town. Suppose that there are three equal-size groups in the economy with the following demand curves: Group...
-
Event services and management can be a lucrative revenue generator. What are the two most important factors in developing a successful event service and management business, whether it is independent...
-
Show how the buying process occurs in the consumer. Review some of the steps in the buying process, stories like: felt need pre-purchase activity purchase decision Post-purchase feelings Explain and...
-
How did Henry Ford set the stage for some of the same problems we still face today in employee relations, especially in manufacturing? 2) If you were a human resources manager, how would you address...
-
What does a DMO risk by not having a positioning theme? Critique the potential of your destination's slogan to effectively differentiate against rivals. you have been asked by a television network to...
-
(a) Construct a Lewis structure for hydrogen peroxide, H2O2, in which each atom achieves an octet of electrons. (b) Do you expect the O-O bond in H2O2 to be longer or shorter than the bond in O2?
-
State whether each of the following will increase or decrease the power of a one-way between-subjects ANOVA. (a) The effect size increases. (b) Mean square error decreases. (c) Mean square between...
-
When 2000 L/min of water flows through a circular section with an inside diameter of 300 mm that later reduces to a 150-mm diameter, calculate the average velocity of flow in each section.
-
Figure 6.16 shows a fabricated assembly made from three different sizes of standard steel tubing listed in Appendix G.2. The larger tube on the left carries 0.072 m 3 /s of water. The tee branches...
-
A standard Schedule 40 steel pipe is to be selected to carry 10 gal/min of water with a maximum velocity of 1.0 ft/s. What size pipe should be used?
-
On April 1, year 1, Mary borrowed $200,000 to refinance the original mortgage on her principal residence. Mary paid 3 points to reduce her interest rate from 6 percent to 5 percent. The loan is for a...
-
Give a numerical example of: A) Current liabilities. B) Long-term liabilities?
-
Question Wonder Works Pte Ltd ( ' WW ' ) produces ceramic hair curlers to sell to department stores. The production equipment costs WW $ 7 0 , 0 0 0 four years ago. Currently, the net book value...

Study smarter with the SolutionInn App


