Use the standard reduction potentials in Table 18.1 To find (a) A metal ion that reduces Ni
Question:
Use the standard reduction potentials in Table 18.1 To find
(a) A metal ion that reduces Ni2+.
(b) A metal ion that can oxidize Cu.
(c) A metal ion that is reduced by Cr2+ but not H2.
Table 18.1 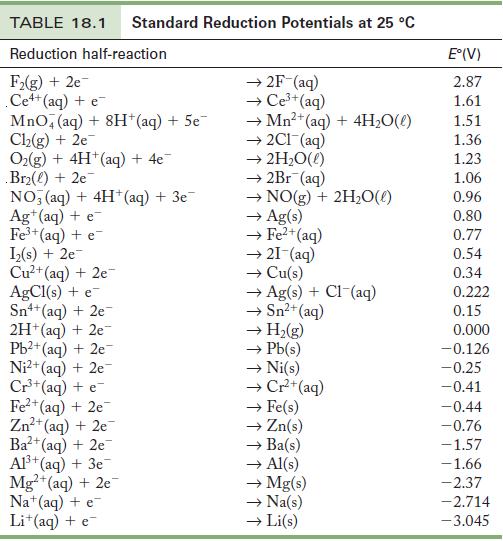
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To answer these questions we must examine the standard reduction potentials in the table you have pr...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Calculate the potential for each of the voltaic cells in Exercise 18.43 when the concentrations of the soluble species and gas pressures are as follows: Exercise 18.43 Use the standard reduction...
-
Write an expression and determine a value for K eq for each voltaic cell in Exercise 18.43 . Exercise 18.43 Use the standard reduction potentials in Table18.1 to find (a) A metal ion that reduces Ni...
-
Use the standard reduction potentials in Table 18.1 to find (a) A reducing agent that will reduce Cu 2+ but not Pb 2+ . (b) An oxidizing agent that will react with Cu but not Fe 2+ . (c) A metal ion...
-
A magnet of mass 5 . 0 1 kg is suspended from the ceiling by a cord as shown. A large magnet is somewhere off to the right, pulling on the small hanging magnet with a constant force of F = 8 0 . 4 N...
-
A spherical satellite in near-earth orbit is exposed to solar irradiation of 1353 W/m2. To maintain a desired operating temperature, the thermal control engineer intends to use a checker pattern for...
-
Zippy motorcycle manufacturing produces two popular pocket bikes (miniature motorcycles with 49cc engines): the Razor and the Zoomer. In the coming week, the manufacturer wants to produce a total of...
-
(Postretirement Benefit Worksheet, Reporting) Dusty Hass Foods Inc. sponsors a postretirement medical and dental benefit plan for its employees. The company adopts the provisions of Statement No. 106...
-
Company R pays $170,000 for a 30% interest in Company E on January 1, 2011. Company Es total stockholders equity on that date is $500,000. The excess price is attributed to equipment with a 5-year...
-
Is there a relationship between volume and strikes resp. maturities?
-
Write an expression and determine a value for K eq for each voltaic cell in Exercise 18.44 . Exercise 18.44 Use the standard reduction potentials in Table18.1 to find (a) A reducing agent that will...
-
Use the data in Appendix H and assume standard conditions when answering the following questions.
-
Prepare (a) The journal entries for 2012 necessary to record the following events and transactions for the College Scholarship Fund; (b) A statement of changes in fiduciary fund net position for the...
-
Vaporization of mixtures of hexane and octane. Using the T-x-y diagram (Figure 1) on the next page, determine the temperature, amounts, and compositions of the vapor and liquid phases at 1 atm for...
-
what should p&g do to replace lafley when he retires a second time? what actions should they take to prepare for the succession?
-
What do these terms mean? What would be the currencies (one at a time) from two total UN Member States (other than the EURO, USD, JPY, GBP, or CHF). What would be the foreign currencies and how they...
-
How do social identity processes, such as categorization, identification, and comparison, influence team cohesion and performance within complex organizational environments ?
-
How do calculate sales forecast and expense forecast for several years
-
(a) Construct a Lewis structure for O2 in which each atom achieves an octet of electrons. (b) Explain why it is necessary to form a double bond in the Lewis structure. (c) The bond in O2 is shorter...
-
A researcher reports a significant two-way between-subjects ANOVA, F(3, 40) = 2.96. State the decision to retain or reject the null hypothesis for this test.
-
If water at 180 F is flowing with a velocity of 4.50 ft/s in a standard 6-in Schedule 40 pipe, calculate the weight flow rate in lb/h.
-
The recommended velocity of flow in the discharge line of an oil hydraulic system is in the range of 8.0 to 25.0 ft/s. If the pump delivers 30 gal/min of oil, specify the smallest and largest...
-
Repeat Problem 6.45, except specify suitable sizes for the suction lines to maintain the velocity between 2.0 ft/s and 7.0 ft/s for 30 gal/min of flow. Repeat Problem The recommended velocity of flow...
-
You plan to buy a house for $325,000 today. If the house is expected to appreciate in value 8% each year, what will its value be seven years from now?
-
A designated beneficiary of an ABLE account must be ___________ in order to meet the special rules that apply to the increased contribution limit authorized under the Tax Cuts and Jobs Act? a. an...
-
Stans wholesale buys canned tomatoes from canneries and sells them to retail markets Stan uses the perpetual inventory

Study smarter with the SolutionInn App


