Write an expression and determine a value for K eq for each voltaic cell in Exercise 18.43
Question:
Write an expression and determine a value for Keq for each voltaic cell in Exercise 18.43 .
Exercise 18.43
Use the standard reduction potentials in Table18.1 to find
(a) A metal ion that reduces Ni2+.
(b) A metal ion that can oxidize Cu.
(c) A metal ion that is reduced by Cr2+ but not H2.
Table 18.1 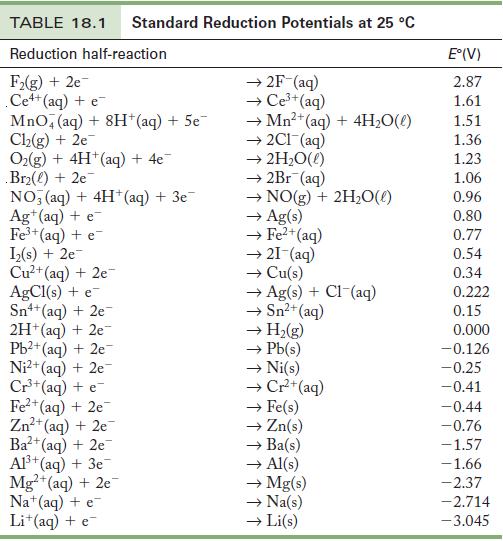
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To determine the value of the equilibrium constant Keq for a voltaic cell you can use the Nernst equ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Write an expression and determine a value for K eq for each voltaic cell in Exercise 18.44 . Exercise 18.44 Use the standard reduction potentials in Table18.1 to find (a) A reducing agent that will...
-
Calculate the potential for each of the voltaic cells in Exercise 18.43 when the concentrations of the soluble species and gas pressures are as follows: Exercise 18.43 Use the standard reduction...
-
Use the standard reduction potentials in Table 18.1 To find (a) A metal ion that reduces Ni 2+ . (b) A metal ion that can oxidize Cu. (c) A metal ion that is reduced by Cr 2+ but not H 2 . Table 18.1
-
A hospital radiology department has the following activities: Activity Number Activity Description 1 Repair X-ray equipment 2 Taking X-ray with X-ray...
-
(a) If the base of the radiator is maintained at Tb = 80C, what is its tip temperature and the rate of heat rejection? Use a computer-based, finite-difference method with a space increment of 0.1 m...
-
What are the main differences between formal and informal reports?
-
General Electric AWall Street Journal article discussed a $1.8 billion charge to income made by General Electric for postretirement benefit costs. It was attributed to previously unrecognized...
-
Enron is a large energy trading company that allegedly committed massive fraud. Enrons primary method of committing fraud was to record liabilities in related partnerships, then known as special...
-
Ratios and Financial Planning at S&S Air, Inc. Chris Guthrie was recently hired by S&S Air, Inc., to assist the company with its financial planning and to evaluate the company's performance. Chris...
-
What is G for the oxidation of metallic iron by dichromate (Cr 2 O 2- 7 ) in acidic solution assuming that the iron is oxidized to Fe 2+ ? Use the data in Appendix H.
-
Use the standard reduction potentials in Table 18.1 to find (a) A reducing agent that will reduce Cu 2+ but not Pb 2+ . (b) An oxidizing agent that will react with Cu but not Fe 2+ . (c) A metal ion...
-
Gronseth Drywall Systems, Inc., is in discussions with its investment bankers regarding the issuance of new bonds. The investment banker has informed the firm that different maturities will carry...
-
1) Why do you believe that in recent years PE sponsors have increasingly chosen to buy debt in their distressed LBOs? 2) What are the pros and cons of this investment strategy? 3) What issues are...
-
Paper Street Soap Company Ltd conducts a business that makes luxury soaps. It operates a factory in Oyster Bay near Sydney. The factory contains a large amount of equipment that is used in the...
-
TRANSACTION ANALYSIS: Dartmouth Ties Corporation is a merchandising company that has been in operation for two years. The company sell high - end ties for men. They purchase their inventory from...
-
Using your knowledge of types of group influence and of subcultures, explain the potential impact on consumer behavior of Methodism's tightening of its ban on gay marriage and LGBTA clergy. Write in...
-
A language L over an alphabet is co-finite, if * \ Lis empty or finite. Let COFNFA = {(N) | N is a NFA accepting a co-finite language}. Show that COF NFA is decidable.
-
(a) What is meant by the term electro negativity? (b) On the Pauling scale what is the range of electro negativity values for the elements? (c) Which element has the greatest electro negativity? (d)...
-
Using the theoretical sampling strategy, how many samples of size 4 (n = 4) can be drawn from a population of size: (a) N = 5? (b) N = 8? (c) N = 16? (d) N = 50?
-
If a pump removes 1.65 gal/min of water from a tank, how long will it take to empty the tank if it contains 7425 lb of water?
-
Calculate the diameter of a pipe that would carry 75.0 ft 3 / s of a liquid at an average velocity of 10.0 ft/s.
-
If the velocity of a liquid is 1.65 ft/s in a special pipe with an inside diameter of 12 in, what is the velocity in a 3-indiameter jet exiting from a nozzle attached to the pipe?
-
A company is evaluating a new 4-year project. The equipment necessary for the project will cost $3,300,000 and can be sold for $650,000 at the end of the project. The asset is in the 5-year MACRS...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
I need to see where the calculations for this problem come from plz. 5. Award: 4.00 points Lucido Products markets two computer games: Claimjumper and Makeover. A contribution format income statement...

Study smarter with the SolutionInn App


