What is the fraction of acid ionized in each acid in Exercise 15.64? Exercise 15.64 Use the
Question:
What is the fraction of acid ionized in each acid in Exercise 15.64?
Exercise 15.64
Use the Ka values in Table 15.6 to calculate the pH of the following solutions.
(a) 0.050 M HI
(b) 0.85 M HF
(c) 0.15 M CH3COOH
(d) 0.017 M C6H5COOH
Table 15.6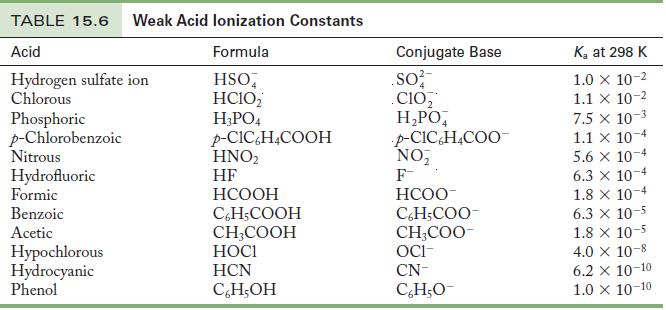
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The fraction of acid ionized often represented as a can be calculated using the ionization constant ...View the full answer

Answered By

Surojit Das
I have vast knowledge in the field of Mathematics, Business Management and Marketing. Besides, I have been teaching on the topics Management leadership, Business Administration, Human Resource Management, Business Communication, Accounting, Auditing, Organizer Behaviours, Business Writing, Essay Writing, Copy Writing, Blog Writing since 2020. It is my personality to act quickly in any emergency situations when students need my services. I am very professional and serious in every questions students asked me at the time of dealing any projects. I have been serving detailed, quality, properly analysed research paper through the years.
4.80+
91+ Reviews
279+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
What is the fraction of acid ionized in each acid in Exercise 15.63? Exercise 15.63 Use the K a values in Table 15.6 to calculate the pH of the following solutions. (a) 0.33 M HNO 2 (b)0.016 M...
-
What is the fraction of acid ionized in each acid in Exercise 15.62? Exercise 15.62 Write the iCe table and set up the equation needed to solve for the concentration of the hydrogen ion in the...
-
What is the fraction of acid ionized in each acid in Exercise 15.61? Exercise 15.61 Write the iCe table and set up the equation needed to solve for the concentration of the hydrogen ion in the...
-
} S 1995 the # of Farms Century, data year 1935 1990 19.50 Aumcon people living on declined steadily during the shown by the Follow g as (in milion of persons) from 1935 19.55 1960 1965 11975 1980...
-
A concentric tube heat exchanger uses water, which is available at 15C, to coo] ethylene glycol from 100 to 60C. The water and glycol flow rates are each 0.5 kg/s. What are the maximum possible heat...
-
Lothar owns a bakery. He has been trying to obtain a long-term contract with the owner of Marthas Tea Salons for some time. Lothar starts an intensive advertising campaign on radio and television and...
-
On May 8, 2008, Jett Company (a U.S. company) made a credit sale to Lopez (a Mexican company). The terms of the sale required Lopez to pay 800,000 pesos on February 10, 2009. Jett prepares quarterly...
-
1. Consider a market with two firms managed by Harry and Vera. Under a cartel (both firms pick the high price), each firm earns a profit of $80. Under a duopoly (both firms pick the low price), each...
-
Calculate the price of the following bonds, where F is the face value, c is the coupon rate, N is the number of years to maturity, and i is the interest rate (or discount rate, or yield). Please show...
-
Write the chemical equation for the ionization of caffeine, a weak base. The chemical formula of caffeine is C 8 H 10 N 4 O 2 .
-
Use the K a values in Table 15.6 to calculate the pH of the following solutions. (a) 0.050 M HI (b)0.85 M HF (c)0.15 M CH 3 COOH (d) 0.017 M C 6 H 5 COOH Table 15.6
-
Suppose that and are of bounded variation on a closed interval [a, b). a) Prove that is of bounded variation on [a, b] for every a R. b) Prove that is of bounded variation on [a, b]. c) If there...
-
Shire Company's predetermined overhead rate is based on direct labor cost. Management estimates the company will incur $649,000 of overhead costs and $590,000 of direct labor cost for the period....
-
You plan to live 25 years after you retire. You want to withdraw $100,000 each year for 25 years. Your first withdrawal will take place the day after you retire. What is the four annuity formulas...
-
Harwood Company's quality cost report is to be based on the following data: 2021 2022 Depreciation of test equipment $94,000 $95,000 Audits of the effectiveness of the quality system $54,000 $51,000...
-
Cash contribution of 4,000 to the Accounting Society (a charity) Purchase of art object at an Accounting Society Charitable event for $1,200 (FMV $800) Donation of 3-year-old clothing (basis 800; FMV...
-
The government is issuing $100 million in 10 year debt and receives the following bids. $25 million is reserved for non-competitive tenders. At what yield will the non-competitive tenders be issued...
-
Which region of the periodic table shown here contains the most readily oxidized elements? Which region contains the least readily oxidized? A
-
A copper sphere of 10-mm diameter, initially at a prescribed elevated temperature T;, is quenched in a saturated (1 atm) water bath. Using the lumped capacitance method, estimate the time for the...
-
This problem involves the design of a parallel adder-subtracter for 8-bit numbers expressed in sign and magnitude notation. The inputs X and Y are in sign and magnitude, and the output Z must be in...
-
Design a multiplier that will multiply two 16-bit signed binary integers to give a 32-bit product. Negative numbers should be represented in 2s complement form. Use the following method: First...
-
The objective of this problem is to use Verilog to describe and simulate a multiplier for signed binary numbers using Booths algorithm. Negative numbers should be represented by their 2s complement....
-
Problem 1 5 - 5 ( Algo ) Lessee; operating lease; advance payment; leasehold improvement [ L 0 1 5 - 4 ] On January 1 , 2 0 2 4 , Winn Heat Transfer leased office space under a three - year operating...
-
Zafra and Stephanie formed an equal profit- sharing O&S Partnership during the current year, with Zafra contributing $100,000 in cash and Stephanie contributing land (basis of $60,000, fair market...
-
What is the Breakeven Point in units assuming a product selling price is $100, Fixed Costs are $8,000, Variable Costs are $20, and Operating Income is $32,000 ? 100 units 300 units 400 units 500 units

Study smarter with the SolutionInn App


