Write the chemical equation and use the data in Tables 15.6 and 15.8 to calculate the base
Question:
Write the chemical equation and use the data in Tables 15.6 and 15.8 to calculate the base ionization constant for the following ions.
(a) Formate ion
(b) Nitrite ion
Tables 15.6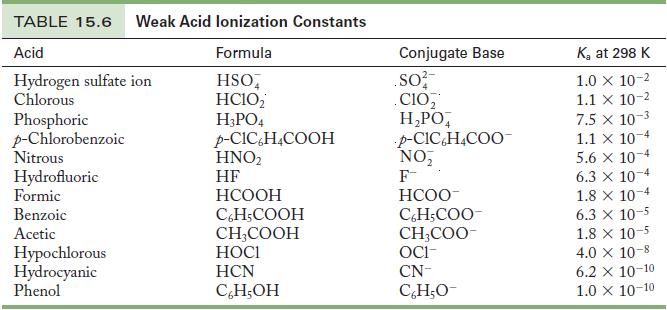
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a HCOO HOHCOOH OH Kw ...View the full answer

Answered By

Mamba Dedan
I am a computer scientist specializing in database management, OS, networking, and software development. I have a knack for database work, Operating systems, networking, and programming, I can give you the best solution on this without any hesitation. I have a knack in software development with key skills in UML diagrams, storyboarding, code development, software testing and implementation on several platforms.
4.90+
97+ Reviews
194+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Is the criterion 6 3CO 2 ) 2 (aq) is found to be 9.11. However, the contribution to the pH from the autoprotolysis of water was ignored. Repeat the calculation of the pH of this solution, taking into...
-
Calcium acetate, Ca(CH 3 CO 2 ) 2 (aq), is used to treat patients with a kidney disease that results in high levels of phosphate ions in the blood. The calcium binds to the phosphates so that they...
-
Although there are extensive tables available for the pK a of weak acids, you might be dealing with an unknown acid or a known acid at an unlisted temperature. You could then use a procedure like...
-
The common stock of Scarlet Enterprises currently pays a quarterly dividend of $1.75 per share. The dividend is assumed to grow annually at a constant rate of 4.0%. The market beta for Scarlet...
-
Consider a concentric tube heat exchanger with hot and cold water inlet temperatures of 200 and 35C, respectively. The flow rates of the hot and cold fluids are 42 and 84 kg/h, respectively. Assume...
-
How do duty-based ethical standards differ from outcome-based ethical standards?
-
On December 31, 2008, Reggit Company held the following short-term investments in its portfolio of available-for-sale securities. Reggit had no short-term investments in its prior accounting periods....
-
Carmeli Instrument Inc. manufactures two products: missile range instruments and space pressure gauges. During January, 50 range instruments and 300 pressure gauges were produced, and overhead costs...
-
True or False: When an investing company owns less than 50 percent of another company, the companies must prepare consolidated financial statements.
-
Write the chemical equation and use the data in Tables 15.6 and 15.8 to calculate the base ionization constant for the following ions. (a) Chlorite ion (b) Fluoride ion Table 15.6 Table 15.8
-
Calculate the [OH - ] and the pH of a 0.024 M methylamine solution; K b = 4.2 10 -4 .
-
Everything else held constant, would you rather depreciate a project with DDB depreciation or deduct it under a Section 179 deduction?
-
Rosita Flores owns Rosita's Mexican Restaurant in Tempe, Arizona. Rosita's is an affordable restaurant near campus and several hotels. Rosita accepts cash and checks. Checks are deposited...
-
Your second task will require you to recover a payload from the conversation. Just need 2.3. Need you to explain step by step, and concept by concept if possible. Use wireshark. Tell me your answer...
-
2. Supply for art sketchbooks at a price of $p per book can be modelled by P <10 S(p) = = textbooks. p3+p+3 p 10 (a) What is the producer revenue at the shutdown point? (b) What is the producer...
-
Patterson Company produces wafers for integrated circuits. Data for the most recent year are provided: Expected Consumption Ratios Activity Driver Wafer A Wafer B Inserting and sorting process...
-
The elementary gas-phase reaction 2A + B C+D is carried out isothermally at 450 K in a PBR with no pressure drop. The specific reaction rate was measured to be 2x10-3 L/(mol-min-kgcat) at 50C and the...
-
Write balanced molecular and net ionic equations for the reactions of (a) Hydrochloric acid with nickel (b) Dilute sulfuric acid with iron (c) Hydrobromic acid with magnesium, (d) Acetic acid,...
-
The first law of thermodynamics is sometimes whimsically stated as, You cant get something for nothing, and the second law as, You cant even break even. Explain how these statements could be...
-
A synchronous 4-bit UP/DOWN binary counter has a synchronous clear signal CLR and a synchronous load signal LD. CLR has higher priority than LD. Both CLR and LD are active high. D is a 4-bit input to...
-
Examine the following Verilog code and answer the following questions module Problem(X,CLK,Z1,Z2); input X,CLK; output Z1,Z2; reg [1:0]State,Nextstate; initial begin State = 2'b00; Nextstate = 2'b00;...
-
(a) Write a behavioral Verilog description of the state machine you designed in Problem 1.13. Assume that state changes occur on the falling edge of the clock pulse. Instead of using if-then-else...
-
Callaho Inc. began operations on January 1 , 2 0 1 8 . Its adjusted trial balance at December 3 1 , 2 0 1 9 and 2 0 2 0 is shown below. Other information regarding Callaho Inc. and its activities...
-
Required: 1. Complete the following: a. Colnpute the unit product cost under absorption costing. b. What is the company's absorption costing net operating income (loss) for the quarter? c. Reconcile...
-
Bond Valuation with Semiannual Payments Renfro Rentals has issued bonds that have an 8% coupon rate, payable semiannually. The bonds mature in 6 years, have a face value of $1,000, and a yield to...

Study smarter with the SolutionInn App


