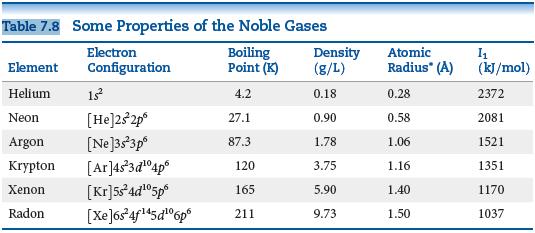
In Table 7.8, the bonding atomic radius of neon is listed as 0.58 , whereas that for
Question:
In Table 7.8, the bonding atomic radius of neon is listed as 0.58 Å, whereas that for xenon is listed as 1.40 Å. A classmate of yours states that the value for Xe is more realistic than the one for Ne. Is she correct? If so, what is the basis for her statement?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:





