Which arrangement of cations (yellow) and anions (blue) in a lattice is the more stable? Explain your
Question:
Which arrangement of cations (yellow) and anions (blue) in a lattice is the more stable? Explain your reasoning.
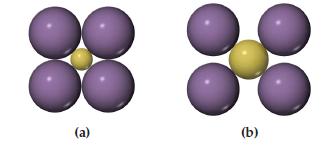
Transcribed Image Text:
(a) (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
Recall that similar charges have strong repulsive tendencies against ea...View the full answer

Answered By

Vijesh J
My passion to become a tutor is a lifetime milestone. Being a finance and marketing professional with hands-on experience in wealth management, portfolio management, team handling and actively contributing in promoting the company. Highly talented in managing and educating students in most attractive ways were students get involved. I will always give perfection to my works. Time is the most important for the works and I provide every answer on time without a delay. I will proofread each and every work and will deliver a with more perfection.
4.70+
5+ Reviews
15+ Question Solved
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:
Students also viewed these Sciences questions
-
Explain why anions are always larger than the atoms from which they are derived, whereas cations are always smaller than the atoms from which they are derived.
-
Consider the two stereo isomers of 3-isoproplcyclohexanol. (a) Which is the more stable conformation of each stereo isomer? (b) Which is the more stable stereo isomer by how much?
-
Name the following compound, identify each substituent as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (yellow-greenC1):
-
Accounts Receivable AED 5,000 Accounts Payable 15,000 Advertising Expense 2,000 16,500 Building Cash Common Stock 50,000 Dividends 1,200 Equipment 2,000 Land 70,000 Notes Payable 60,000 3,400 Office...
-
Oxygen at 100 kPa and 200oC is compressed to half its initial volume. Determine the final state in terms of pressure (p2) and temperature (T2) if the compression is carried out in an (a) Isobaric....
-
Describe evidence supporting the following types of coding in STM: auditory (Conrad letter memory experiment); visual (Della Sala matrix experiment); and semantic coding (Wickens fruits and...
-
What do you mean by chargeable expenses?
-
The owner of an automobile repair shop studied the waiting times for customers who arrive at the shop for an oil change. The following data with waiting times in minutes were collected over a 1-month...
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year . A...
-
There is a lottery with n coupons and n people take part in it. Each person picks exactly one coupon. Coupons are numbered consecutively from 1 to n, n being the maximum ticket number. The winner of...
-
The electronic structure of a doped semiconductor is shown here. (a) Which band, A or B, is the valence band? (b) Which band is the conduction band? (c) Which band consists of bonding molecular...
-
Two solids are shown below. One is a semiconductor and one is an insulator. Which one is which? Explain your reasoning.
-
Find formulas for the functions represented by the integrals. -2x sec tdt
-
4. What is the time complexity of the following procedure for in/2 to n do j 2 end for while (j
-
If the concentration of a constituent in the influent to the equalization basin is constant over the 24 h period, will the load of the constituent from the basin be constant? If the concentration of...
-
A three-phase transmission line of a 60 Hz circuit has a length of 370 km (230 miles). the conductors are of the 795,000cm (54/7) type with horizontal spacing of 25 feet between them. The load on the...
-
Simulate rolling a dice using Math.random() . Your roll function should allow the caller to specify any number of sides, but default to 6 if no side count is given: roll() assumes a 6 sided dice,...
-
Drama Read the excerpt from a play. Then, answer the question(s). (1) (2) Belle: Having trouble deciding what will make you look like both a power to be reckoned with and a fetching young lady while...
-
A block pushed along the floor with velocity v 0x slides a distance d after the pushing force is removed. a. If the mass of the block is doubled but its initial velocity is not changed, what distance...
-
You've been asked to take over leadership of a group of paralegals that once had a reputation for being a tight-knit, supportive team, but you quickly figure out that this team is in danger of...
-
How does a trigonal pyramid differ from a tetrahedron so far as molecular geometry is concerned?
-
Describe the bond angles to be found in each of the following molecular structures: (a) Planar trigonal, (b) Tetrahedral, (c) Octahedral, (d) Linear.
-
(a) What is meant by the term electron domain? (b) Explain in what way electron domains behave like the balloons in Figure 9.5.Why do they do so? Two balloons linear orientation Three balloons...
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


