The first three energy levels (E(mathrm{eV})) of the fictitious element (mathrm{X}) are (ldots . . ldots) shown
Question:
The first three energy levels \(E(\mathrm{eV})\) of the fictitious element \(\mathrm{X}\) are \(\ldots . . \ldots\) shown in Figure P29.56.
a. What wavelengths are observed in the absorption spectrum of element X? Give your answers in \(\mathrm{nm}\).
b. State whether each of your wavelengths in part a corresponds to ultraviolet, visible, or infrared light.
c. An electron with a speed of \(1.4 \times 10^{6} \mathrm{~m} / \mathrm{s}\) collides with an atom of element X. Shortly afterward, the atom emits a \(1240 \mathrm{~nm}\) photon. What was the electron's speed after the collision? Assume that, because the atom is so much more massive than the electron, the recoil of the atom is negligible.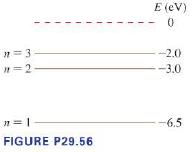
Step by Step Answer:

College Physics A Strategic Approach
ISBN: 9780321907240
3rd Edition
Authors: Randall D. Knight, Brian Jones, Stuart Field





