The diagram below shows the mass of measuring cylinder before some liquid is poured into it and
Question:
The diagram below shows the mass of measuring cylinder before some liquid is poured into it and then after.
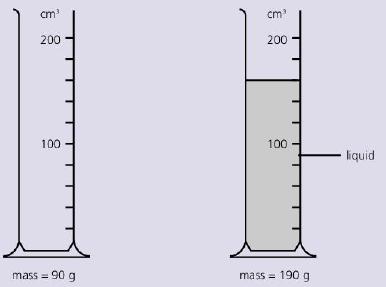
What is the density of the liquid?
A. 100/160 g/cm3
B. 100/130 g/cm3
C. 190/160 g/cm3
D. 100/130 g/cm3
Transcribed Image Text:
cm cm 200 200 100 100 liquid mass = 90 g mass = 190 g !!
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
Option A is Correct answer Density Mass Volume ...View the full answer

Answered By

Muhammad Waqas Hanif
I have experience of 2 years teaching. Also I have sound knowledge of research. I teach students at High School Level to Master Level.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The diagram below shows the various short-run cost curves for a perfectly competitive firm. a. Based on the diagram, and the assumption that the firm is maximizing its profit, fill in the table. The...
-
The diagram below shows the Canadian market for leather shoes, which we assume to be competitive. The world price is pw. If the Canadian government imposes a tariff of t dollars per unit, the...
-
The diagram below shows the short run demand curve (D), marginal revenue curve (MR), average total cost curve (ATC), and marginal cost curve (MC) for a firm in a monopolistically competitive market....
-
Suppose the given numbers for a mental calculation (see Problem 59) are 10x + y and 10x + z. Notice that these two numbers have the same tens digit. Also assume that y + z = 10, which says that the...
-
Use the VSEPR model to predict the geometry of the following ions: a. N3 b. BH4 c. SO32 d. NO2
-
Consider the following network for conducting a two week (10 working days) computer training class: (a) Construct a schedule showing: ESs for all activities LSs for all activities Slacks for all...
-
Why is it necessary for strict regulations to be in force on the casino floor?
-
Consider again Problem 13. The point of purchasing a European option is to limit the risk of a decrease in the per-share price of the stock. Suppose you purchased 200 shares of the stock at $28 per...
-
The Giuntoli Co. just issued a dividend of $3.00 per share on its common stock. The company is expected to maintain a constant 5 percent growth rate in its dividends indefinitely. If the stock sells...
-
A part-time employee who rolls out dough balls at a pizza restaurant was observed over a 40-hour period for a work-sampling study. During that time, she prepared 550 pieces of pizza dough. The...
-
Isotopes of the radioactive element uranium occur naturally in small proportions in some rocks. The table gives information about one uranium isotope. a. How many neutrons are there in an atom of...
-
Drops of water from a cracked gutter fall past the window of an IGCSE Physics students room, as shows in the diagram. The student uses a digital stopwatch to find the time between one drop and the...
-
A hospital has 125 deciliter bags of blood plasma. What is the volume of plasma expressed in milliliters? Strategy Plan STEP 1: What unit is asked for in the answer? STEP 2: What given value is...
-
Scenario : Wanda, a BCBA, is updating an intervention plan for a leaner on her caseload to submit for insurance funding authorization. Part of the plan includes the completion of an adaptive...
-
a) how can your company accommodate generational or gender difference within your company ? b) how can your company accommodate communication and or language difference within your company ?
-
3. Consider the following data for two catalysts, A and B. The temperature is 25 C and the reaction occurs at standard conditions. a. Make a Tafel plot and determine the Tafel slope. Estimate the...
-
How consumption can be helpful in facilitating the construction of your identity? Explain the ways in which the symbolic meanings, connected with your consumption choices are important to you? Is...
-
Cataumet Boats, Inc. Jaime Giancola had just completed the first half of her MBA program and wanted to work on a project during the summer that would give her some practical experience applying what...
-
On September 30, 2019, Rolen Machinery Co. sold a machine and accepted the customers zero-interest-bearing note. Rolen normally makes sales on a cash basis. Since the machine was unique, its sales...
-
Let (x) = x 2 - 9, g(x) = 2x, and h(x) = x - 3. Find each of the following. (((--) 2
-
While 13 C is the main contributor to the (M+1) + peak, there are many other elements that can also contribute to the (M+1) + peak. For example, there are two naturally occurring isotopes of...
-
Below are mass spectra for four different compounds. Identify whether each of these compounds contains a bromine atom, a chlorine atom, or neither. a. b. c. d. 100- 80- 60- 60- 40 20- 0- 60 70 10 20...
-
For each of the following molecules, determine the number of carbon atoms present, and then determine the number of hydrogen atoms connected to each carbon atom: a. b. c. d. e. f. g. h. i. j. k. l....
-
Long-term liabilities are shown in two places in the business firm's balance sheet depending upon when the long-term liabilities are scheduled for payment. True False
-
Julio is single with 1 withholding allowance. He earned $1,025.00 during the most recent semimonthly pay period. He needs to decide between contributing 3% and $30 to his 401(k) plan. If he chooses...
-
Acquirer firm plans to launch a takeover of Target firm. The manager of Acquirer indicates that the deal will increase the free cash flow of the combined business by $13.6m per year forever. The beta...

Study smarter with the SolutionInn App


