Prepare debtors and creditors control accounts for the year commencing 1 July 2015 from the following information:
Question:
Prepare debtors and creditors control accounts for the year commencing 1 July 2015 from the following information:
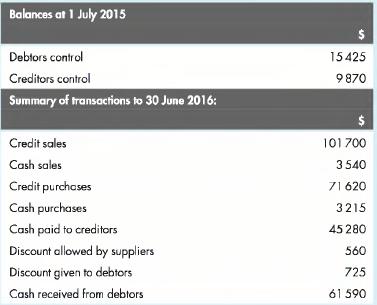
Transcribed Image Text:
Balances at 1 July 2015 Debtors control Creditors control Summary of transactions to 30 June 2016: Credit sales Cash sales Credit purchases Cash purchases 15425 9870 101700 3540 71 620 3215 Cash paid to creditors Discount allowed by suppliers 45 280 560 725 Cash received from debtors 61590 Discount given to debtors
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Certainly Lets prepare the Debtors Control Account and the Creditors Control Account based on the in...View the full answer

Answered By

Raunak Agarwal
Teaching is my hobby and now my profession. I teach students of CA and CFA(USA) in batches of 100 students and have a 5 year experience.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Financial Accounting An Integrated Approach
ISBN: 9780170349680
6th Edition
Authors: Ken Trotman, Michael Gibbins, Elizabeth Carson
Question Posted:
Students also viewed these Business questions
-
Prepare debtors and creditors control accounts for the year commencing 1 July 2018 from the following information: Balances at 1 July 2018 Debtors' control Creditors' control Summary of transactions...
-
In view of the information provided below provide suggestions for the efficient management of trade debtors. INFORMATION MANAGEMENT OF TRADE DEBTORS Debtor management is central to the effective cash...
-
Maurice Deleon operates an Electrical Supplies business. He has a large number of debtors and creditors and maintains both Debtors and Creditors Control accounts. The following information was taken...
-
Greety Food in Ashland, Kentucky, manufactures and markets snack foods. Sita Lee manages the company's fleet of 220 delivery trucks. Lee has been charged with *reengineering* the fleet-management...
-
Extend Prob. 3.46 to the problem of computing the center of pressure L of the normal face Fn, as in Fig. P3.122. (At the center of pressure, no moments are required to hold the plate at rest.)...
-
The work of I/O psychologists and how it benefits organizations and employees alike
-
Suppose Dominos wants to open a new restaurant. What are some secondary sources of information that might be used to conduct research on potential new locations? Describe how these sources might be...
-
Describe the differences between general-use software and generalized audit software. How might you use spreadsheet software, database software, and word processing software in conducting an audit of...
-
Way Cool produces two different models of air conditioners. The company produces the mechanical systems in its components department. The mechanical systems are combined with the housing assembly in...
-
Preparing an Individuals Tax Form. Caleb Lee graduated from college in 2018 and began work as a systems analyst in July of that year. He is preparing to file his income tax return for 2018, and has...
-
Jupiter Ltd uses multi-column cash receipts and cash payments journals, and maintains control accounts for accounts receivable and accounts payable, supported by subsidiary ledgers. Balances in the...
-
James Stewart owns a general store in a country town. He keeps two subsidiary ledgers (an accounts receivable ledger and an accounts payable ledger) and a general ledger. Balances in the subsidiary...
-
Discuss which of the dimensions of the environment brings about the greatest challenge for management.
-
16. List I describes four systems, each with two particles A and B in relative motion as shown in figures. List II gives possible magnitude of their relative velocities (in m s) at time t = 3 S....
-
17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible changes in the internal energy of the...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
You visited the foreign exchange trading room of a major bank when a trader asked for quotes of the euro from various correspondents and heard the following: Bank A 1.1210-15 Bank B 12-17 What do...
-
A regular deposit of $100 is made at the beginning of each year for 20 years. Simple interest is calculated at i% per year for the 20 years. At the end of the 20-year period, the total interest in...
-
Beaver Construction purchases new equipment for $50,400 cash on April 1, 2024. At the time of purchase, the equipment is expected to be used in operations for seven years (84 months) and have no...
-
The general ledger of Jackrabbit Rentals at January 1, 2024, includes the following account balances: The following is a summary of the transactions for the year: 1. January 12 Provide services to...
-
Consider the following transactions for Huskies Insurance Company: 1. Income taxes for the year total $42,000 but wont be paid until next April 15. 2. On June 30, the company lent its chief financial...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


