Consider the waste treatment process shown in the figure below. In this process, wastewater containing a dissolved
Question:
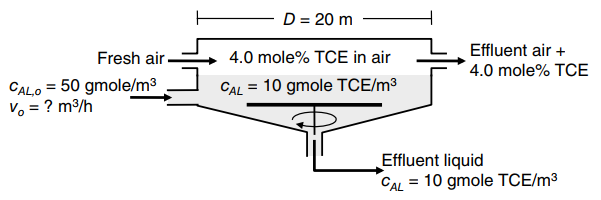
a. What is the overall mass-transfer coefficient based on the liquid phase, as KL?
b. What is the flux of TCE from the clarifier liquid surface?
c. Develop a well-mixed, steady material balance model for the process. What is the inlet volumetric flow rate of wastewater, vo (in units of m3/hr) needed to ensure that the liquid effluent TCE concentration is cAL = 10 gmole TCE/m3?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster
Question Posted:





