A 0.1475-M solution of Ba(OH) 2 was used to titrate the acetic acid (60.05 g/mol) in a
Question:
A 0.1475-M solution of Ba(OH)2 was used to titrate the acetic acid (60.05 g/mol) in a dilute aqueous solution. The following results were obtained.
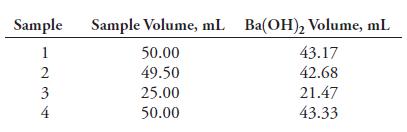
(a) Calculate the mean w/v percentage of acetic acid in the sample.
(b) Calculate the standard deviation for the results.
(c) Calculate the 90% confidence interval for the mean.
(d) At the 90% confidence level, could any of the results be discarded?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:





