Analysis of several plant-food preparations for potassium ion yielded the following data: The preparations were randomly drawn
Question:
Analysis of several plant-food preparations for potassium ion yielded the following data:
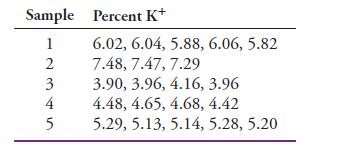
The preparations were randomly drawn from the same population.
(a) Find the mean and standard deviation s for each sample.
(b) Obtain the pooled value spooled.
(c) Why is spooled a better estimate of σ than the standard deviation from any one sample?
Transcribed Image Text:
Sample Percent K+ 6.02, 6.04, 5.88, 6.06, 5.82 7.48, 7.47, 7.29 3.90, 3.96, 4.16, 3.96 4.48, 4.65, 4.68, 4.42 5.29, 5.13, 5.14, 5.28, 5.20 1 N345
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Analytical Chemistry
ISBN: 9780357450390
10th Edition
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
Question Posted:
Students also viewed these Sciences questions
-
Find the mean and standard deviation of four-digit uniformly distributed lottery numbers (0000 through 9999).
-
For this data set, find the mean and standard deviation of the variable. The data represent the serum cholesterol levels of 30 individuals. Count the number of data values that fall within 2 standard...
-
For this data set, find the mean and standard deviation of the variable. The data represent the ages of 30 customers who ordered a product advertised on television. Count the number of data values...
-
Smart Price Company, a producer of black forest cakes, has budgeted sales and production (in units) for the last quarter in 2019 to be as follows: Sales Production October November 26,000 28,000...
-
Construct the complete sinking fund schedule. Calculate the total interest earned by adding up the interest earned column and by calculating the difference between the final balance in the fund and...
-
Draw a box-and-whiskers display for the set of data with the 5-number summary 4262728297.
-
Support for an entrepreneurs project. Does displaying positive emotions (e.g., joy) during a funding pitch help an entrepreneur gain more financial support? This was the question of interest in a...
-
Dobler Company just took its physical inventory on December 31. The count of inventory items on hand at the companys business locations resulted in a total inventory cost of $300,000. In reviewing...
-
-Buy Aircraft sells a wide variety of model aircrafts and uses a perpetual inventory system. On June 1 -Buy Aircraft had five Cessna 560 model airplanes on hand at a unit cost of $105.00. During June...
-
1. Liam Richardson is the business manager for the Smith & Lyngate Insurance agencies in the state of Maryland. Liam is interested in increasing the number of agents in Baltimore and plans to buy...
-
Before agreeing to the purchase of a large order of solvent, a company wants to see conclusive evidence that the mean value of a particular impurity is less than 1.0 ppb. What hypotheses should be...
-
Determination of phosphorous in blood serum gave results of 4.40, 4.42, 4.60, 4.48, and 4.50 ppm P. Determine whether the 4.60 ppm result is an outlier or should be retained at the 95% confidence...
-
A force F of magnitude 320 N acts at the origin of a coordinate system. Knowing that x = 104.5 Fz = 120 N, and Fy < 0, determine (a) The components Fx and Fy, (b) The angles y and z.
-
5.Descibe the HSI color image model 6. Describe the basic relationship between the pixels
-
1. What is the need for transform? 2. What is Image Transform? 3. What are the applications of transform? 4. Give the Conditions for perfect transform . 5. What are the properties of unitary...
-
6. Define Fourier transform pair 7. Define Fourier spectrum and spectral density 8. Give the relation for 1-D discrete Fourier transform pair 9. Specify the properties of 2D Fourier transform. 10....
-
16. What is wrap around error? 17. Give the formula for correlation of 1D continuous function. 18. What are the properties of Haar transform. 19. What are the Properties of Slant transform 20....
-
21. Define fast Walsh transform. 22. Give the relation for 1-D DCT. 23. Write slant transform matrix SN. 24. Define Haar transform. 25. Define K-L transform. 26. Give the equation for singular value...
-
Are financial statement audits intended to detect fraud?
-
Continuation of Exercise 4-83. (a) What is the probability that the first major crack occurs between 12 and 15 miles of the start of inspection? (b) What is the probability that there are no major...
-
Calculate the molar solubility of SrC2O4 in a solution that has a fixed H3O+ concentration of (a) 1.0 10-6 M. (b) 1.0 10-9 M.
-
Calculate the molar solubility of BaSO4 in a solution in which [H3O+] is (a) 3.5 M. (b) 0.080 M.
-
Calculate the molar solubility of PbS in a solution in which [H3O+] is held constant at (a) 3.0 10-1 M and (b) 3.0 10-4 M.
-
At a 3% (EAR) rate of interest, you will quadruple (increase four folds) your money in approximately ____ years.
-
Smile Company makes baked goods. The budgeted sales are $620,000, budgeted variable costs are $260,400, and budgeted fixed costs are $237,800. What is the budgeted operating income?
-
Analysis of a replacement project At times firms will need to decide if they want to continue to use their current equipment or replace the equipment with newer equipment. In this case, the company...

Study smarter with the SolutionInn App


